Author
Hiroyasu Yokomise, MD.,Ph.D. Professor, Department of General Thoracic Surgery,
Director of Kagawa University Hospital, Vice President of Kagawa University
Biography
Graduation from the Faculty of Medicine, Kyoto University in 1981. Research fellow at the Division of Thoracic Surgery, University of Toronto (Canada) for one year from 1988, after work at the Department of Thoracic Surgery of Kurashiki Central Hospital and Department of Cardiovascular Surgery of National Himeji Hospital. Assistant at the Department of Thoracic Surgery, Chest Disease Research Institute, Kyoto University in 1991. Professor of the Second Department of Surgery at Kagawa Medical University in 1999, after work at the Department of Thoracic Surgery of the Japanese Red Cross Wakayama Medical Center. Professor of Department of General Thoracic Surgery at Kagawa University since 2003, and the Director of the Kagawa University Hospital since 2014, specializing in surgical therapies for advanced lung cancer as well as in reconstructive surgery.
Introduction
The number of cases of lung cancer indicated for segmentectomy is increasing for the following reasons.
1) Increase in the number of small lung cancers expecting radical surgery
2) Minimally invasive surgery for elderly lung cancer patients
3) Limited resection for patients with poor pulmonary function
4) Radical resection of the entire lesion for synchronous multiple lung cancers
5) Preservation of pulmonary functions for metachronous multiple lung cancers
While the standard surgery for lung cancer is lobectomy or wider resection and dissection of the hilar-mediastinal lymph nodes, we must wait for the results of the currently ongoing clinical study to decide whether segmentectomy can be a standard procedure for lung cancer.
Until then, we, as chest surgeons, should perform lung segmentectomy in way that minimizes complications and local recurrence.
Segmentectomy not based on correctly identified anatomical borders of lung segments may result in residual intrapulmonary regional lymph nodes and postoperative recurrence, late-onset pulmonary fistula, etc.
In addition, a sufficient margin is required for oncological curability.
For these reasons, when performing segmentectomy for lung cancer, it is possible to prevent surgical complications and improve curability by identifying the intersegmental borders accurately. We have demonstrated in animal experiments that it is possible to visually identify the anatomical borders of lung segments by using infrared thoracoscopy (IRT) combined with the use of indocyanine green (ICG)1).
Moreover, considering that many lung cancer surgeries are currently performed thoracoscopically, the use of the conventional inflation- deflation method to identify intersegmental borders is less than ideal in terms of safety and speed. To enable rapid and safe surgery, we have been developing IRT combined with the use of ICG in collaboration with Olympus1)-6). In addition, it should be noted that the application of ICG for fluorescent imaging during thoracic surgery is currently off- label use.
Methods and Results
ICG absorbs light in the near infrared region and emits fluorescence at 845nm by irradiation of excitation light with peak at around 805nm.
Because ICG is metabolized in the liver and excreted in the bile without affecting organs, it is highly safe. However, since it is an iodine agent, clinicians need to be aware if their patients are allergic to iodine. Since segmental pulmonary arteries run parallel to segmental bronchi, pulmonary blood flows are dynamically visualized by blocking the pulmonary artery blood flow in the relevant segment and administering ICG by rapid intravenous injection under IRT. This allows us to identity the location of intersegmental borders precisely. The intersegmental borders visualized about 20 seconds after intravenous injection are marked using electrocautery.
The characteristics of the two types of IRT system that we have reported on so far are shown below.
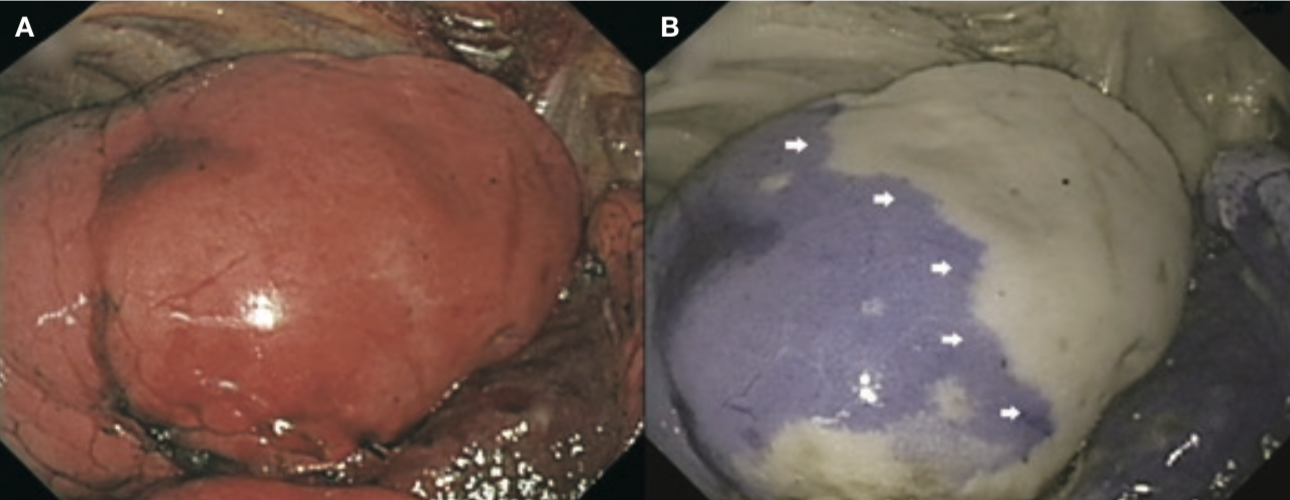
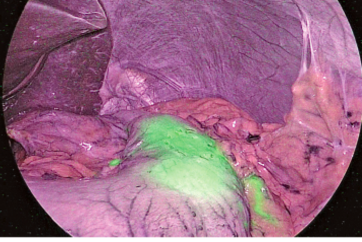
1. The optical absorption method was used in a two-wavelength model with irradiation of near infrared light at different wavelengths of 805 and 940nm, respectively. Using signal processing, the IRT system displays the 805nm and 940nm light in red and blue, respectively. The 805nm light is attenuated in areas containing ICG and displayed in blue on the monitor, while areas not containing ICG are displayed in white on the monitor (Figure 1). The dose of ICG required was 3.0mg/kg, and the mean duration of ICG staining was 210 seconds (3.5 minutes).
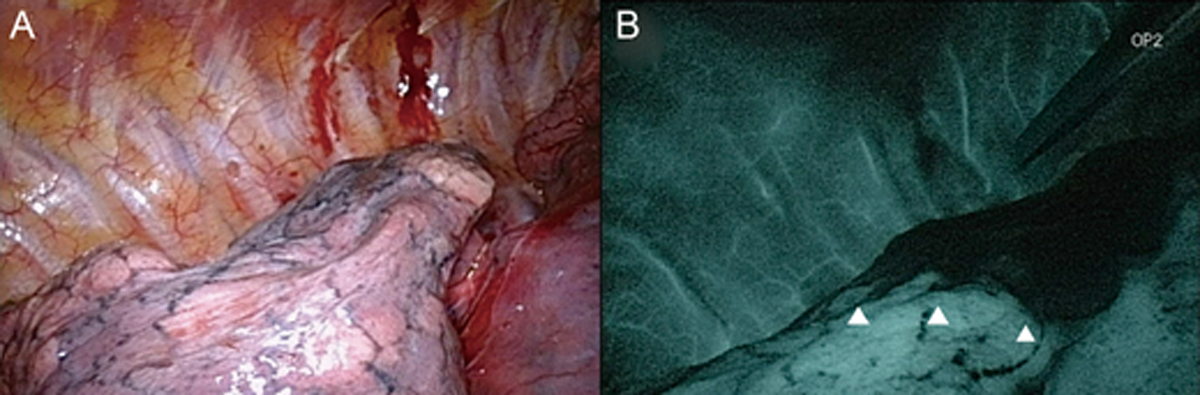
2. The fluorescence method was used in a single-wavelength model.
The areas containing ICG irradiated with near infrared light emit fluorescence at 845nm and are shown as white on a darkened monitor (Figure 2). The dose of ICG required was 0.5mg/kg, and the mean duration of ICG staining was 393 seconds (6.55 minutes).
The optical absorption method improves the quality of surgery by allowing the surgeon to observe the entire visual field and the instruments. However, it has a drawback in that a large amount of ICG is required for administration but persists for a short duration.
Using the fluorescence method, we had to perform the procedure under darkened conditions. This made it difficult to see the positional relationships between the surgical field and instruments.
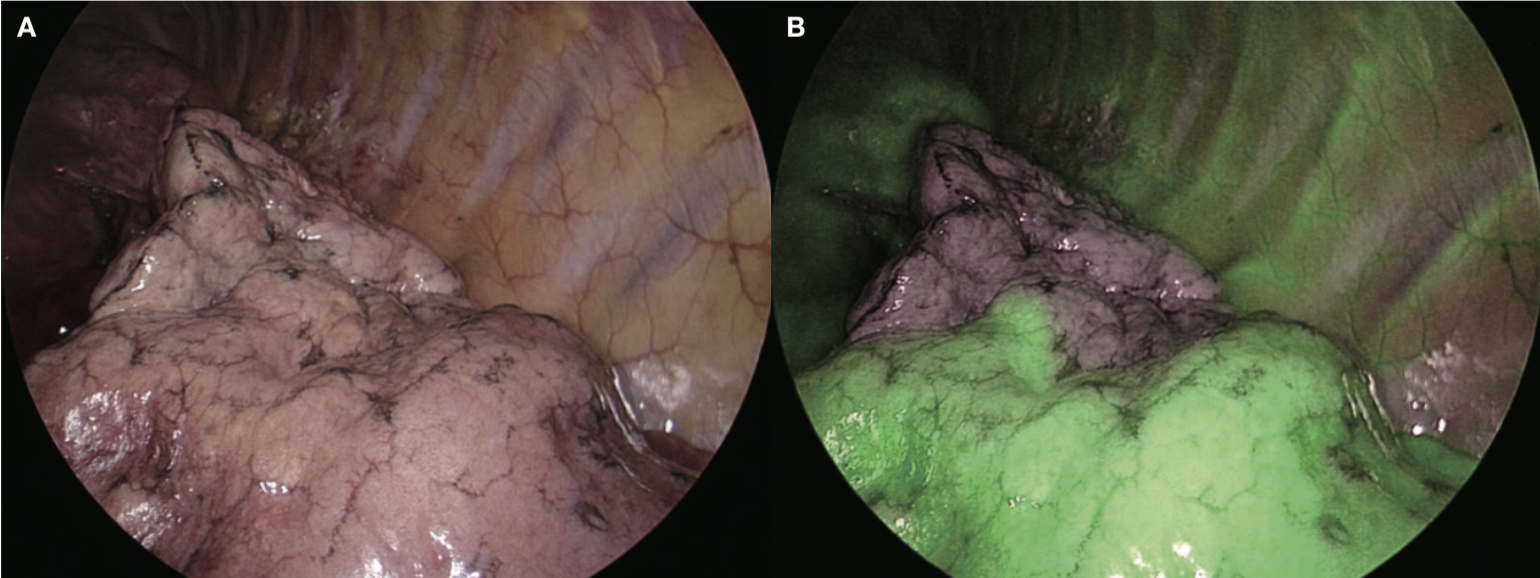
New IRT system
The new IRT system, VISERA ELITE II, uses a fluorescence method that is similar to the single-wavelength model. However, thanks to simultaneous irradiation of the reference light in addition to the excitation light, observation of the whole field is made possible by also illuminating the areas that are not emitting fluorescence, thus providing good surgical visibility, which is a characteristic of the two-wavelength model (Figure 3). In our case, ICG was administered at 0.1-0.3mg/kg, which persisted for 5 minutes or longer, showing the advantage of the fluorescence method. As this makes it possible to check the entire field and instruments, marking can be done safely and securely. It is possible to switch between normal light observation and IR observation using a nearby switch, and the size and weight (220g) of the camera head do not markedly differ from those of the conventional system, providing good manageability. This system will undoubtedly play an important role in developing secure, safe and rapid segmentectomy in the future.
References
- Misaki N, Yokomise H et al. J Thorac Cardiovasc Surg 2009;138:613-8
- Gotoh M, Yokomise H et al. J Thorac Cardiovasc Surg 2003;126:1916-21
- Gotoh M, Yokomise H et al. J Thorac Cardiovasc Surg 2007;134:1498-501
- Misaki N, Yokomise H et al. J Thorac Cardiovasc Surg 2010;140:752-6
- Kasai Y, Yokomise H et al. Eur J Cardiothorac Surg 2013;44:1103-7
- Tarumi S, Yokomise H et al. Eur J Cardiothorac Surg 2014;46:112-5
- Keyword
- Content Type