Cytology and Histotyping
Author: Birgit. Guldhammer-Skov, MD, DrMSci, Bispebjerg Hospital, Copenhagen University Hospital, Denmark
Source: DVD-ROM ‘Endoscopic Ultrasound – Diagnostics and Staging of Lung Cancer’, Olympus Europa SE & Co. KG, 2013
Introduction
The diagnostic value of an EBUS-TBNA and EUS-FNA depends equally on the excellence of the clinical procedure executed to secure the sample, the laboratory procedures used to process the specimen, and the nterpretation done by an experienced (cyto) pathologist. As compared to histological material (i.e. mucosa biopsies and core needle biopsies), there are advantages of cytologic material as obtained by a minimally invasive procedure like EBUS-TBNA and EUS-FNA: the preparation in the laboratory is fast allowing for faster reporting, including onsite assessment, and it is relatively inexpensive.
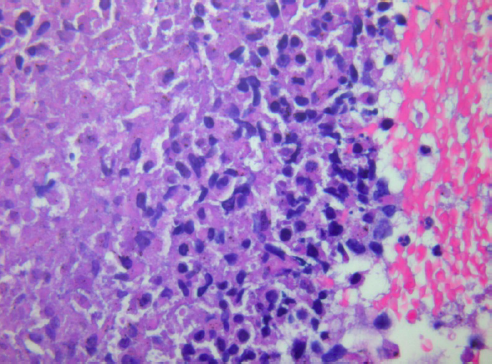
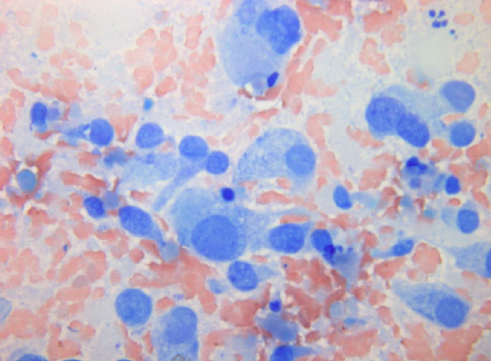
Furthermore, cytology smears do not have formalin-fixation artifacts that can limit interpretation of some biopsies; therefore, cytology frequently provides greater nuclear and cytoplasmic resolution than histology (images 1 and 2).
The accuracy of the cytological examination depends greatly on the quality of the collection, preparation, and staining of the material. The pathologist should be provided with proper information including whether the target is a lymph node (with specific indication of station) or a tumor. If the aspirated lesion might be a metastasis from a known tumor outside the thorax this must be relayed to the pathologist unless the information is already available from a central or national pathology database. The size of an aspirated lymph node can be important for the pathologist as a sparse material might be representative for a very small lymph node, whereas this will not be the case if the aspirate is from an enlarged lymph node. Inadequacy in any of these steps will adversely affect the quality of the cytologic diagnosis.
Cytology is a powerful tool in the diagnosis of cancer, including lung cancer. The reproducibility and the accuracy in separation of small cell lung cancer (SCLC) from non-small cell lung cancer (NSCLC) are excellent. This has great clinical relevance since these diseases should receive different treatment. With the advent of specific therapies for squamous cell carcinoma and adenocarcinoma it has become clear that there is also a need for improved subclassification of NSCLC with separation of these two entities. The need to determine eligibility for molecular testing has made this need even more acute. Despite the lack of conventional architecture, there are rigorous cytology-specific diagnostic criteria and the distinction between squamous cell carcinoma and adenocarcinoma on cytologic material is very good and can be further strengthened by adding immunohistochemistry (IHC) to morphological assessment. In a recent study, it was shown that cytologic subtyping of adenocarcinomas versus squamous cell carcinomas in a practice with routine utilization of IHC is highly feasible with a 3% unclassified rate and accurate with 96% concordance with resection diagnosis (Rekhtman, N., et al. “Suitability of thoracic cytology for new therapeutic paradigms in non-small cell lung carcinoma: high accuracy of tumor subtyping and feasibility of EGFR and KRAS molecular testing.” J.Thorac.Oncol. 6.3 (2011): 451-58.)
Thus, utilizing all available material from the cytological sample in an optimal way, including smears for morphological evaluation and paraffin-embedded material (cell block preparation) for ancillary methods including IHC is of utmost importance and results in high diagnostic accuracy and sensitivity.
Preparation of smears
No matter how expertly the aspiration is performed, if the slides are not interpretable, the procedure is worthless for cytological diagnosis. An ideal aspirate is of creamy consistency with numerous cells suspended in a small amount of tissue fluid without admixture of blood. A prerequisite for making a clinically relevant diagnosis on aspirated material is optimal spread of the cells and preservation of a cell block for IHC and molecular studies.
For the preparation of smears, single-end frosted slides should be utilized. Slides should be labeled with the patient’s ID prior to aspiration. To prepare smears, place the bevel of the needle directly on the glass slide near the frosted end. Express the aspirated material using the air-filled syringe to blow out material through the needle onto the slide in one drop (see image 3).
The actual method will be determined in part by the nature of the material present. If the aspirated material is abundant and fluid the material is easily expressed without force. If minimal tissue particles are present then pick them up for direct smearing (“cyto-imprint”) on another glass. This can usually be done without destroying the material (see image 4).
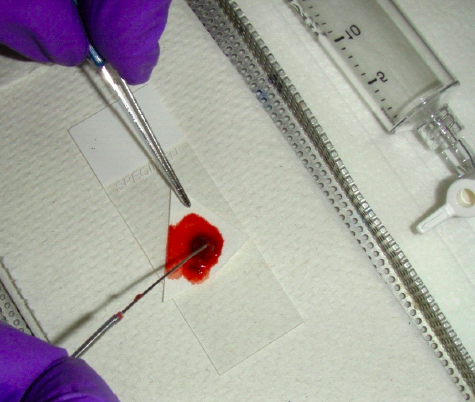
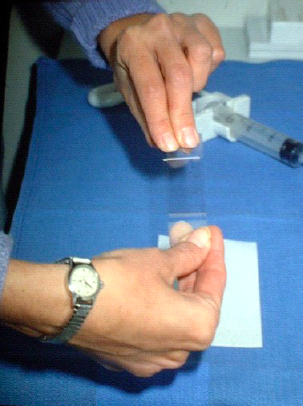
Thereafter the particles should be fixed in formalin for cell block preparation and handled as a histological biopsy. This material is very useful for ancillary studies including IHC and mutation analyses. If the aspirated material is sparse the material must often be expelled with a light pressure using the air initially introduced into the syringe. The former method allows for better control of the smear process. Once the specimen is on the slide, it must be smeared. A second or spreader slide is used to make the smear (image 5).
The spreader slide is gently lowered, crosswise, over the droplet, which will then spread out slightly by capillary action. The spreader slide is then gently pulled straight back in one smooth motion, down the length of the diagnostic slide (image 6).
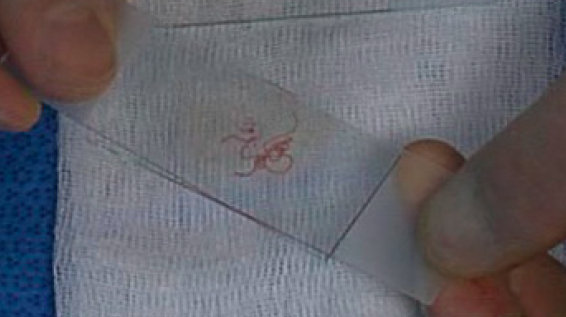
Larger particles can be crushed gently by firm flat pressure. Undue pressure can result in crush / smearing artifact. The smear should not go to the edges of the slide, where particles can be lost over the edges. The outline of a perfect smear is completely contained on the slide without going to any of the edges. The cells must be delicately and thinly smeared with minimal distortion and fixed according to the stain to be used (see below). However, spreading the cells too thinly by using high pressure may cause severe injury making interpretation of the cells impossible. Obtaining optimal smear is a fine balance between too thick and too thin smears (or fixation and crush artifacts) and comes with experience (smearing technique is better demonstrated than described). See an example for a representative smear (stained using MGG) from material obtained by EBUS-TBNA in image 7.
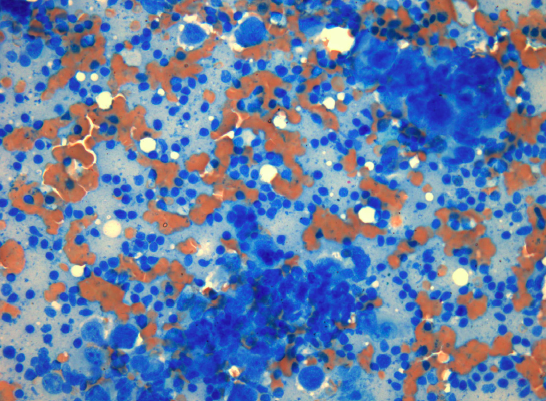
Little or no diagnostic material should remain on the spreader slide. The number of smears is not standardized but we recommend 2-4 per aspiration. Unsuited smears (see images 8 and 9) can be due to non-representative / inadequate samples or due to poor quality of preparation (thick smears, extreme admixture with blood, delayed fixation, over-staining). Attention to matters of technique regarding the procedure and preparation of smears will considerably reduce the number of unsuited smears received in a cytology lab.
Smears which have been exposed to formalin or formalin fumes cannot be used for evaluation so keep formalin away from smears!
The macroscopic appearance of the aspirate can already give some diagnostic information: Grayish, finely granular material and necrotic material is highly suspicious for malignancy (rarely inflammation). Mucoid material is suspicious for a mucoid tumor. Small clumps of tissue may present an aspirate from mesenchymal as well as from epithelial tumor. Thin liquid may represent a cyst, and pus represents infection. Thin and bloody material is often non-diagnostic. The yield of cells depends on the nature of the lesion, aspiration technique, experience of the endoscopist, number of aspirates, and cooperation of the patient.
Staining of smears
Several methods apply to the staining of smears obtained by EBUS-TBNA and EUS-FNA.
1. Air-drying followed by stains like May-Grunwald-Giemsa (MGG) and Diff Quik, Giemsa. In this method, smears are intentionally air dried. If smears are not correctly made and dried quickly, artifacts will result. One advantage is the speed with which smears can be stained especially with use of rapid stains like Diff Quik (2- 3 minutes). Rapid stains are particularly useful in preliminary assessment of the adequacy of the sample before the endoscopy procedure is concluded (see below). Colloid, mucin and endocrine cytoplasmic granules are best brought out in air-dried preparations (see image 10, typical MGG staining).
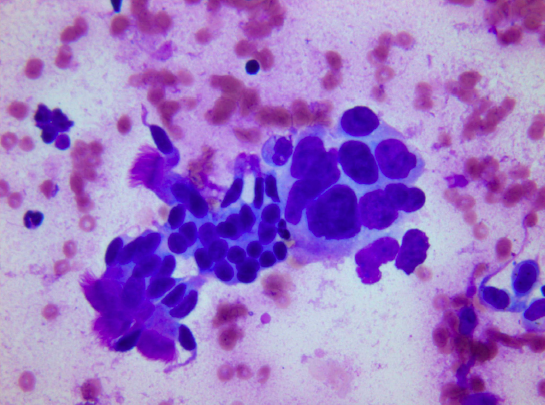
2. Alcohol fixation followed by Papanicolaou (Pap) or hematoxylin and eosin (H&E) Staining. Rapid fixation in alcohol (wet fixation) is essential for Pap staining, which brings out nuclear details clearly, allowing better identification of malignant cells. Smears must be quickly made and fixed otherwise drying artifact can occur in which case the cytoplasm takes up more eosin (red color) and nuclear details are less clear. A cellular sample can become unfit for diagnosis if there is significant drying. At the beginning of the smearing process, while the material is still in a drop on the slide, the surface area for evaporation is relatively small and hence a short delay will not cause significant air-drying. Once the smear has been made, the surface area is greatly increased and the thickness of the smear diminished. From the instant the smear is made, air-drying proceeds extremely rapidly. Hence with Pap staining, air-drying is avoided as much as possible by dropping the slides into the fixative immediately after the smears are made. Poor quality of preparation, fixation or staining can all make a cellular sample unsuited for evaluation (see image 11, typical Pap staining).
Probably due to fixation artifacts, cell crowding and cell overlapping is found to be high with the Pap method as compared to the air-dried method making interpretation difficult as one useful criterion for malignancy in cytological specimens is the three-dimensional arrangement of the malignant cells. Cytoplasm details are better preserved in MGG than in Pap stain. Crisp chromatin granularity is preserved in Pap stain, whereas nuclear chromatin transparency is less in MGG. Thus the limitation of one method can be counterbalanced by the other. The two methods may be regarded as complementary. Familiarity with both stains is indispensable in specific situations. Pathologist should make their own decisions on their accumulated experience.
Diff-Quick staining is primarily used to evaluate the aspirated material on site to make sure that the sampling is adequate (rapid on site evaluation, ROSE). This was especially relevant when lymph nodes were aspirated without ultrasound. In this setting the endoscopist made a blind sampling not knowing if the target had been hit. However, with EUS-FNA and/or EBUS-TBNA available the need for on site evaluation has declined. EUS-FNA/EBUSTBNA allows the visualization of the target structures and operators usually know when the target has been hit rendering ROSE unnecessary in many cases. Furthermore, onsite evaluation requires the pathologist or cytotechnician to be available at any time and to be present in the operating theater which is very time consuming and may not be adequately reimbursed. Perhaps most important, however, is the fact that having an evaluation on site saying that the aspirate is adequate and representative (most often with cells from a lymph node), may lead to no more material being obtained with the risk of having too little material for ancillary methods, such as IHC and molecular testing.
Cell block preparation
After the content of the syringe has been expelled there will usually still be material left in the syringe which can be extracted by saline and made into a clot (image 4). Cell block preparation from the clot is done in the usual way as for biopsies, i.e. fixation in formalin and embedding in paraffin, and should be used in combination with the smears to improve the diagnostic information. The clot can provide additional material for special studies such as IHC and molecular testing. IHC on conventional smears is often non-conclusive because of unspecific background staining of disrupted cells and membrane fragments sticking to the slides.
There are different methods of cell block preparation. With the plasma embedded method, the aspirated material is expelled into a tapered centrifuge tube containing formalin and saline. After spinning the pellet is re-suspended in plasma to which thromboplastin is added and a clot is formed. If the aspirated material is bloody a clot may be formed spontaneously and the material is transferred directly into formalin for fixation. If no material is left in the syringe to make a clot directly, it is possible to make one from the spread material. In short, after the cytology slides have been read, the cells are gently scraped off the slides with a surgical blade. No destaining is necessary prior to this procedure. Clots are made by taking the cells from the blade and putting them into tissue cassettes covered by Millipore paper. In cases with very sparse material 3 drops of human plasma and one drop of thrombin can be added to the cells to form a clot.
The cell blocks, whether prepared from the clot in the syringe or from the scrape clot, should be cut in 2-4μm sections and handled as histological material for ancillary studies. As an example the differentiation between low differentiated adenocarcinoma and squamous cell carcinoma may be difficult on smears alone.
A small panel of immunohistochemical antibodies including TTF1, P63, Napsin and CK5/6 provide highly diagnostic information in most of these cases. In cases with a potential metastatic tumor other makers such as CDx2, CK20, PSA and ER should be applied. Strategic use of cytology samples is important, i.e. use the minimum material necessary for an accurate diagnosis, to preserve as much tissue as possible for potential molecular studies.
With the emerging importance of molecular diagnostics to guide therapy, an approach is needed to preserve tissue samples optimized to perform studies such as DNA sequence analysis, fluorescence in situ hybridization (FISH), and, in some settings, RNA-based studies. Cytological samples can be used for many molecular analyses. EGFR mutation testing and KRAS mutation testing are readily performed on formalin fixed paraffinembedded samples such as cell blocks. Furthermore, previously stained smears can be used for these analyses by simply scraping the cells from the glass directly for DNA extraction and further analyses. The results are comparable to those of surgical biopsy material.
Alternatively, liquid-based monolayer techniques (ThinPrep) can be used for ancillary methods. Ideally, the cytospin should be a monolayer of cells with a minimum of 100 tumor cells per slide.
A small panel of immunohistochemical antibodies including TTF1, P63, Napsin and CK5/6 provide highly diagnostic information in most of these cases. In cases with a potential metastatic tumor other makers such as CDx2, CK20, PSA and ER should be applied. Strategic use of cytology samples is important, i.e. use the minimum material necessary for an accurate diagnosis, to preserve as much tissue as possible for potential molecular studies.
With the emerging importance of molecular diagnostics to guide therapy, an approach is needed to preserve tissue samples optimized to perform studies such as DNA sequence analysis, fluorescence in situ hybridization (FISH), and, in some settings, RNA-based studies. Cytological samples can be used for many molecular analyses. EGFR mutation testing and KRAS mutation testing are readily performed on formalin fixed paraffinembedded samples such as cell blocks. Furthermore, previously stained smears can be used for these analyses by simply scraping the cells from the glass directly for DNA extraction and further analyses. The results are comparable to those of surgical biopsy material.
Alternatively, liquid-based monolayer techniques (ThinPrep) can be used for ancillary methods. Ideally, the cytospin should be a monolayer of cells with a minimum of 100 tumor cells per slide.
Ultra short guidelines for reading cytological material
In experienced hands, reading cytology is not more difficult than reading histology. However, as mentioned previously, representativeness of the sample and high quality of the preparation are the two fundamental requirements on which the success of cytological material depends. If these prerequisites are only partly fulfilled, definitive conclusions may be hard to draw.
A very brief introduction to reading EBUS-TBNA and EUS-FNA cytological specimens follows:
The presence of at least 40 benign-appearing lymphocytes (without any granulocytes present) in a high-power field (HPF: area visible under the maximum magnification power of the objective being used) in the areas of highest cellularity of the smear constitutes an adequate sample. Also a large number of anthracotic pigment-laden macrophages or large clusters of admixed lymphocytes and pigmented macrophages are indicators of adequacy.
Signs of malignancy are:
- loss of uniformity,
- variation of size and shape of cells and nuclei,
- enlargement of nuclei and nucleoli,
- abnormal mitotic figures,
- loss of cohesion of cells,
- necrosis.
Difficult cytological diagnoses are among others highly differentiated carcinomas, low grade lymphomas, low grade sarcomas, rare and unusual tumours, and certain cystic tumors.
There are several pitfalls in reading cytological specimens:
Attention should be given to
- misinterpretation of contaminating cells from luminal surfaces,
- misinterpretation of cells from lymphatic hyperplasia,
- very few malignant cells present,
- tumor cells with only a few signs of malignancy,
- sub-optimal material (bloody, diluted, artifacts).
Proper use of EUS-FNA and EBUS-TBNA requires close communication between an experienced (cyto) pathologist and an experienced clinician. The pitfalls listed above may be avoided by providing more information to the cytopathologist
- by describing the needle path,
- by providing several samples from a lesion (consecutive aspirates),
- by preparing smears with the optimal spreading technique,
- by adding clinical information.
In turn the cytopathologist should advise on the most suitable method for preparing cyto smears or artificial cell blocks in order to receive the material in the most suitable condition.
Most importantly increasing practice and experience in fine needle aspiration will help to improve reading from cytology slides and to reduce the risks listed above.
How to increase knowledge of reading cytology slides
Intensive courses, lead by experienced (cyto)pathologists, for inexperienced pathologists to quickly improve their diagnostic accuracy. By combining the opportunity to read a large number of diagnostics smears (simple cases mixed up with rare ones) by themselves with sessions of multi-head microscopy lead by the experienced pathologist (demonstrating easy cases as well as subtle details), steep learning curves can be achieved.
- Content Type