Gastrointestinal scope series
Clinical Advantages of Gastrointestinal Endocyto for Diagnosis of Esophageal Lesions
Dr. Haruhiro Inoue
Showa University Koto Toyosu Hospital, Digestive Diseases Center
Endocyto can also be used as a routine scope for the upper gastrointestinal lesions
Magnifying endoscopic observation of the esophageal mucosa was once considered to have no clinical significance. However, in the mid-1990s we identified and reported on the intraepithelial papillary capillary loop (IPCL) under magnifying observation1). We then discovered that the IPCL shows characteristic morphological changes in esophageal intraepithelial cancers, and published a report2). Since then, changes in the IPCL have been widely recognized as an important indicator reflecting the grade of tissues atypia in the endoscopic diagnosis of esophageal cancer. Once the interpretation of magnifying endoscopy was completed, the next goal was direct in vivo observation of cancer cells. Accordingly, with Kumagai3), Inoue4), and Oue et al. we have been proceeding with the development of ultra-high magnification endoscopy using a flexible scope, in collaboration with Olympus, since the first half of the 2000s. After a long development process, the Endocyto gastrointestinal scope has now become available as a product featuring not only conventional narrow-band imaging (NBI) and magnifying observation functions but with the additional function of ultra- high magnification (endocytoscopy: EC) (Figure 1). The Endocyto can be used for screening endoscopy because its diameter (tip diameter of the technology verifier model: 9.7 mm) is even smaller than that of the GIF-H290Z, a magnifying endoscope model that can be used routinely for upper gastrointestinal endoscopy. In other words, it can first be used for [1] screening by non-magnifying observation to locate a lesion, followed by [2] tissue characterization by magnifying NBI observation (conventional magnifying observation after locating the lesion), and [3] in the case of suspected cancer, EC (cell observation) at even higher magnifications to obtain additional nuclear-level information for diagnosis.
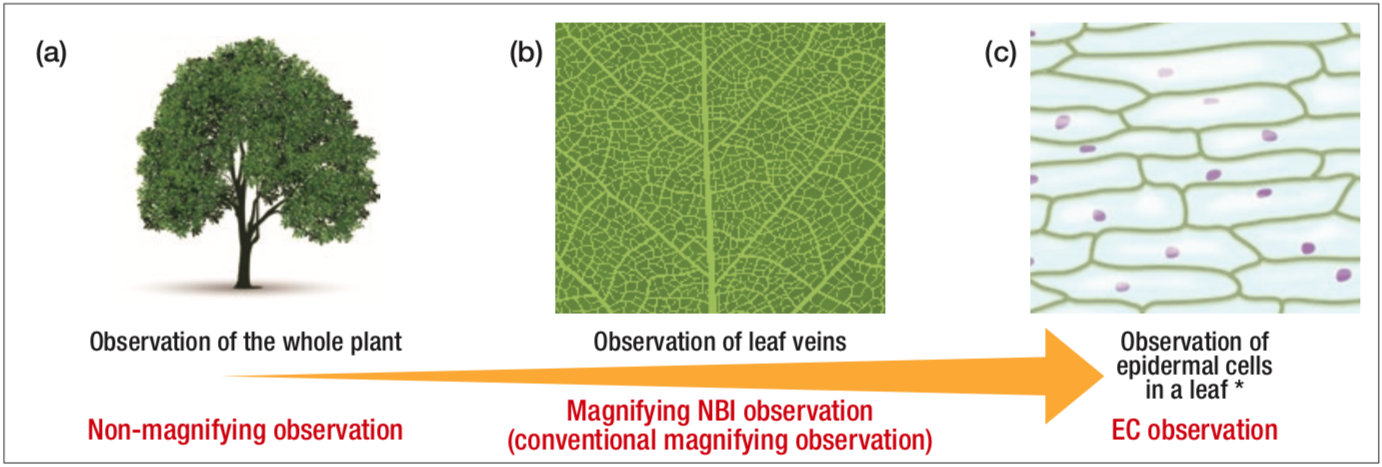
Figure 1
Conceptual diagram showing that non-magnifying observation, magnifying NBI observation, and endocytoscopy can be performed by using Endocyto alone
* The illustration shows a diagram of an observation image of scale leaf of onion
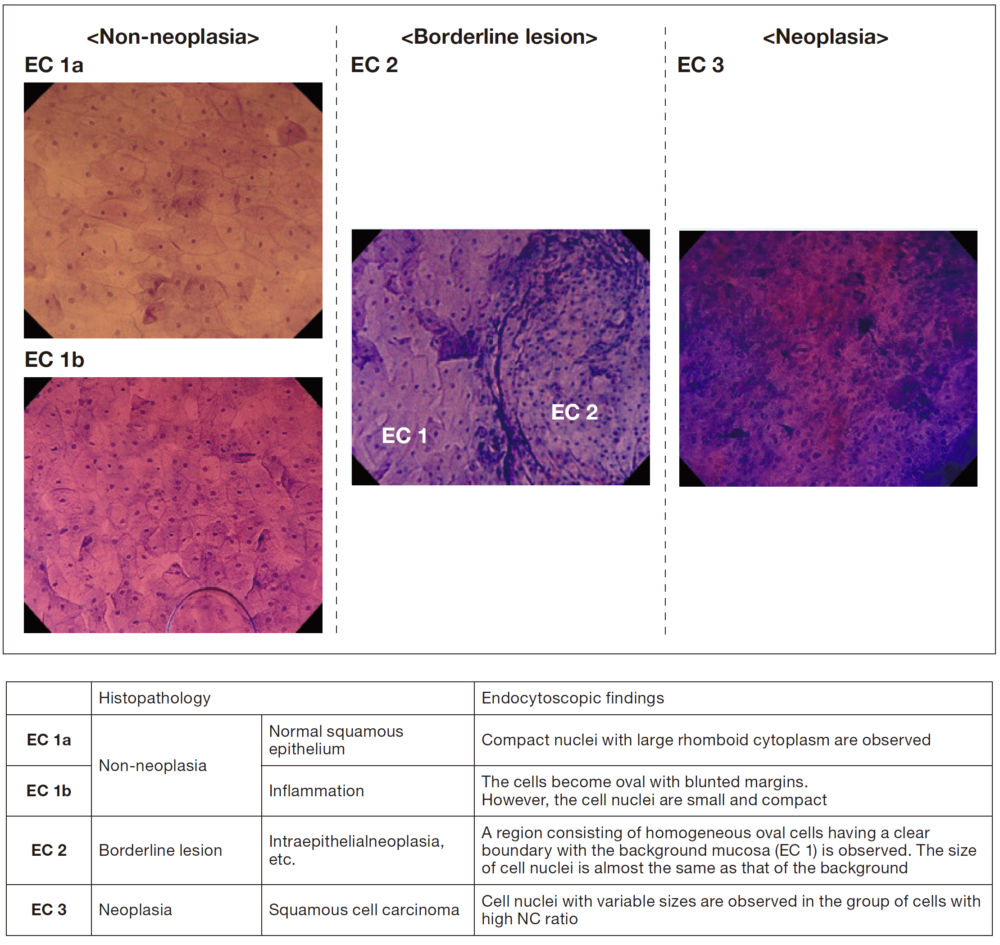
Additional diagnostic information for differentiation between neoplasia and non-neoplasia in the esophagus
For EC observation of the esophagus, double staining with crystal violet and methylene blue (hereafter referred to as CM staining)5), which stains nuclei in blue and cytoplasm in pink, provides images similar to those obtained by hematoxylin-eosin (HE) staining, allowing visualization of cells and nuclei in the most superficial layer of the squamous epithelium. I consider EC to be most useful for differentiating between neoplasia and non-neoplasia in endoscopic diagnosis of borderline lesions in the esophagus. However, for diagnosis of cancer invasion depth when having to choose between endoscopic treatment or surgery, a highly accurate diagnosis including evaluation of the extent of submucosal invasion can be made by capturing morphological changes in the IPCL (extent of microvascular destruction) using conventional magnifying NBI observation6). For these reasons, we use the EC classification with three categories (EC 1, 2, and 3) corresponding to non-neoplasia, borderline lesion, and neoplasia, respectively (Figure 2), to classify the EC findings that are indicators for endoscopic diagnosis of the esophagus7). I consider that this EC classification for the esophagus, which is based on the endocytoscopic atypia (ECA) classification consisting of five categories that we initially proposed8), is simpler and suitable for application in clinical practice, as these three categories are consistent with EC classification of the colorectum9).
In the current situation, the standard method for confirming a differential diagnosis between neoplasia and non-neoplasia consists of performing biopsy and histopathological diagnosis of the sampled specimen. Considering that histopathological diagnosis using biopsy specimens allows us to perform immunostaining in addition to HE staining, which stains nuclei and cells, such a diagnosis certainly provides a greater amount of information compared with EC images, which can only be stained by CM. However, the endoscopic diagnosis using Endocyto provides comprehensive differentiation between neoplasia and non-neoplasia by adding information on the EC findings to the basic diagnostic information including the findings of non-magnifying observation, magnifying NBI observation, and any trend toward an increase in lesion size compared with the previous examination.
Unnecessary biopsies can be reduced by improving the ability of endocytoscopy
In the future, owing to the dissemination of EC and accumulation of experience, I expect that diagnostic ability of EC comparable with that of diagnostic biopsy can be achieved for differentiating between neoplasia and non-neoplasia in the esophagus. In addition, development of a computer-aided diagnostic system using EC images is currently ongoing10, 11), the practical application of which may further improve the diagnostic ability of EC. If as a consequence it becomes possible to omit biopsy and arrive at a definitive diagnosis of neoplasia/non-neoplasia by endoscopic examination alone, many clinical advantages can be expected.
Especially in the context of the aging of the population in recent years, we are increasingly concerned about the bleeding risk associated with biopsy because of the increasing proportion of patients taking antithrombotic drugs. The potential to omit unnecessary biopsies and minimize the number of specimens to be collected by EC in these patients would be a great advantage in terms of patient safety. Moreover the workload of pathologists, which is currently excessive, may be alleviated.
In addition, compared with the esophagus and colorectum, it is difficult to obtain well-stained images in the stomach when EC is used, which is an important problem to be surmounted if it is to be established as a clinically useful diagnostic technique. However, if the aforementioned computer-aided diagnostic system is realized in the future, the lack of image quality can be compensated for by the computer-aided pattern recognition, and thus it may become possible to perform a highly accurate diagnosis without problems, even in the stomach.
Expected development of endoscopic diagnostics associated with utilization of Endocyto
In the future, endoscopists using Endocyto will quickly arrive at a definitive diagnosis by adding EC observation findings to conventional non-magnifying observation and magnifying NBI observation as diagnostic information and by verifying the consistency among these data in a series of processes for endoscopic diagnosis. I expect this to lead to further development of endoscopic diagnostics in general.
[References]
1) Inoue H, Honda T, Yoshida T, et al. Ultra-high magnification endoscopy of the normal esophageal mucosa. Dig Endosc 1996; 8: 134-138.
2) Inoue H, Honda T, Nagai K, et al. Ultra-high magnification endoscopic observation of carcinoma in situ of the esophagus. Dig Endosc 1997; 9: 16-18.
3) Kumagai Y, Monma K, Kawada K. Magnifying chromoendoscopy of the esophagus: in-vivo pathological diagnosis using an endocytoscopy system. Endoscopy 2004; 36: 590-594.
4) Inoue H, Kazawa T, Sato Y, et al. In vivo observation of living cancer cells in the esophagus, stomach, and colon using catheter-type contact endoscope, “Endo-Cytoscopy system”. Gastrointest Endosc Clin N Am 2004; 14: 589-594.
5) Minami H, Inoue H, Yokoyama A, et al. Recent advancement of observing living cells in the esophagus using CM double staining: endocytoscopic atypia classification. Dis Esophagus 2012; 25: 235-241.
6) Inoue H. Magnification endoscopy in the esophagus and stomach. Dig Endosc 2001; 13: S40-S41.
7) Haruhiro Inoue. Endocytoscopy. Hisao Tajiri (Editor): NBI/BLI/LCI Endoscopic Atlas Based on New Criteria and Classifications. 100-105. Nihon Medical Center, 2016.
8) Inoue H, Sasajima K, Kaga M, et al. Endoscopic in vivo evaluation of tissue atypia in the esophagus using a newly designed integrated endocytoscope: a pilot trial. Endoscopy 2006; 38: 891-895.
9) Kudo SE, Wakamura K, Ikehara N, et al. Diagnosis of colorectal lesions with a novel endocytoscopic classification-a pilot study. Endoscopy 2011; 43: 869-875.
10) Misawa M, Kudo SE, Mori Y, et al. Characterization of colorectal lesions using a computer-aided diagnostic system for narrow-band imaging endocytoscopy. Gastroenterology 2016; 150: 1531-1532.
11) Mori Y, Kudo SE, Wakamura K, et al. Novel computer-aided diagnostic system for colorectal lesions by using endocytoscopy (with videos). Gastrointest Endosc 2015; 81: 621-629.
- Keyword
- Content Type