Faculty
Gregory B. Haber, MD
Professor, Department of Medicine
Chief of Endoscopy
Director of Advanced Therapeutics and Innovation NYU Langone Medical Center
New York、New York
Stephen J. Heller, MD
Professor, Department of Medicine Lewis Katz School of Medicine
Director of Endoscopy
Temple University Hospital
Philadelphia, Pennsylvania
Covered Versus Uncovered Stents for Malignant Biliary Strictures
Researchers at the Cleveland Clinic concluded that the efficacy and safety data provide “a compelling argument” for choosing uncovered stents for managing malignant biliary strictures.1 Covered biliary stents were initially developed to reduce the risk for tumor tissue ingrowth posed by uncovered stents, and thereby improve luminal patency duration.2 Indeed, early studies appeared to support an advantage for covered stents.3,4 Subsequent luminal patency and clinical data, however, indicate comparable efficacy between both stent types.1,5,7 In addition to demonstrating comparable efficacy, recent research shows that covered stents are associated with significantly higher rates of migration, pancreatitis, and cholecystitis (Table).1,5
“We hoped that covered stents would obviate the problem of tissue ingrowth, provide longer luminal patency times, and result in fewer adverse events, but the medical research has not borne that out. Covered stents do not seem to provide a significant advantage,” said Stephen J. Heller, MD, the director of endoscopy at Temple University Hospital, in Philadelphia, Pennsylvania.1,4-8 “You get less tumor ingrowth because covered stents are impenetrable, but they migrate more frequently. That’s a serious problem; when a stent slips out of position, it no longer keeps the obstructed duct open for drainage and may migrate toward the bowel or liver, where it may be difficult to retrieve. Impaired drainage of the cystic duct also can lead to cholecystitis, and the same is true of the pancreatic duct and pancreatitis,” Dr Heller added. “Neither of these complications are as common as migration, but they can be serious.”
“There has been a shift toward using uncovered stents for malignant disease,” said Gregory B. Haber, MD, the chief of endoscopy and director of advanced therapeutics and innovation at NYU Langone Medical Center, in New York, New York. “Covered stents are mandatory for benign disease to allow for easy removal.9 However, if the obstruction is at the hilum or higher up, you can really only use uncovered stents because they allow branched ducts to drain into the common bile duct.”
Ideal Stent Properties
Selecting an effective biliary stent entails more than a consideration of stent type, Dr Haber said. “You want sufficient radial force to expand within the cancer and provide adequate luminal patency, low axial force to provide the necessary stability for adaptation to the contour of the duct, and a range of sizes to match stent length to the tumor that you’re trying to open up.”
Ample radial force expands the stent, thereby fixating it to the stricture site, for more effective dilation and thus drainage of the bile duct.10 Conversely, high axial force—a greater tendency of the stent to straighten rather than conform to the contours of the bile duct—is associated with bile duct impaction, pancreatitis, and cholecystitis.10,11
Dr Haber said an effective stent also should minimize shortening as it expands in the bile duct because an undeployed stent with a greater degree of shortening is more difficult to position for release. “When the obstruction is high up in the bile duct, accurate positioning can be more challenging if the pre-deployment stent is 30% to 40% longer than the deployed stent,” he said.
Table. Key Results of 2 Retrospective Cohort Studies Comparing Clinical Outcomes Of Uncovered Versus Covered Biliary Stents in Patients With Malignant Biliary Stricture
| Outcome | All | Uncovered | Covered | P Value |
| Jang et al, 2018 | ||||
| Clinical success, % | 92.4 | 92.1 | 93.0 | 0.69 |
| Patency duration, d | 551.8 | 557.9 | 546.7 | 0.14 |
| Cholecystitis, % | 3.1 | 1.2 | 7.8 | <0.001 |
| Stent migration, % | 4.5 | 1.4 | 10.7 | <0.001 |
| Lee et al, 2013 | ||||
|
Recurrent obstruction at 1 y, % |
37.0 | 38.0 | 35.0 | 0.61 |
|
Overall survival at 1 y, % |
48.0 | 49.0 | 45.0 | 0.84 |
| Pancreatitis, % | 2.0 | 1.0 | 6.0 | <0.001 |
| Stent migration, % | 10.0 | 2.0 | 36.0 | <0.001 |
Based on references 1 and 5.
Advantages of a Unique Design
Lumina/ Patency
The HANAROSTENT Biliary uncovered stent from Olympus America Inc (Figure 1) is designed for ease of use and to maximize luminal patency duration. The uniquely braided hook-cross nitinol design provides optimal radial and axial force.12 In addition, nitinol (vs steel) is associated with longer patency times and a lower incidence of stent failure.13
“The HANAROSTENT Biliary hook and cross design offers a major advantage in that it creates a strong radial force for expansion while reducing the axial force, which increases flexibility and allows the stent to conform to the anatomy of the bile duct, “Dr Haber said. “That’s a feature unique to this stent; it offers conformability to the tumor, but not at the expense of adequate expansion. The hook and cross design also means that the foreshortening ratio is lower, so it is easier to position the stent, especially when you’re dealing with a malignant obstruction higher up in the bile duct, ” he added.
Dr Heller agreed. “Some other stents shorten once they go in, which is a problem when it shortens to the point that it’s no longer effective. This stent does not shorten as much、 making it a more reliable device. The lower axial force makes it less traumatic to the surrounding tissue, and the optimal radial force keeps the bile duct lumen open, but not so much that it stretches the tissue too much, which can cause unnecessary pain.”
Placement and Targeting
Other features include 12 radiopaque markers on the proximal, distal, and middle of the stent to facilitate placement under fluoroscopy and a range of sizes, including a commonly used 6-cm stent as well as new 5- and 7-cm length options, that target strictures of various sizes to meet the unique needs of the patient.12
“The radiopaque markers are very visible, which makes it easy to position the stent, and increases accuracy,” Dr Haber said. ” The HANAROSTENT comes in a larger number of lengths compared with other stents on the market, giving you more flexibility. You can choose a stent length to match the size of the obstruction you’re dealing with instead of being restricted.”
“You want the stent to be long enough to cross the stricture, but if it’s too long it can cause blockage;’Dr Heller added. “The additional lengths allow me to deliver a more personalized, precise stent that suits the patient and the length of the stricture, and it’s helpful that the HANAROSTENT has radiopaque markers on the stent itself,” he said. “Other stents have markers on the deployment system, so when you’re using an x-ray machine to deploy the stent, you’re using surrogate markers for positioning. Having the markers right on the stent allows for more accurate deployment.”
If the stent appears to be in a suboptimal position, it can be retrieved and repositioned until a “point of no return,” indicated in red on the device handle.12 “You can pull the sheath back over the stent, recapture it, and start over, which is helpful,” Dr Heller said. He also noted the flared ends of the HANAROSTENT prevent migration.12 “The flared ends are a nice feature; uncovered stents have a lower risk for migration compared with covered stents, but the flared ends may lower that risk even further”.1,5
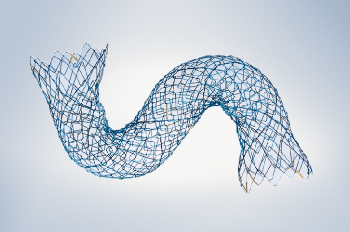
Figure 1. Flexible deployed stent
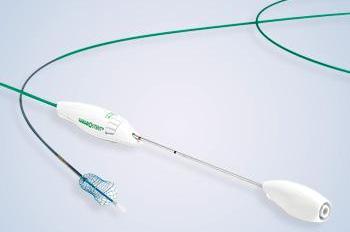
Figure 2. Full deployment system with stent
Conclusion
Although covered stents are appropriate for benign disease, the antimigration properties and superior safety profile of uncovered stents make them the superior choice for managing malignant biliary strictures1,5,11 The HANAROSTENT Biliary uncovered stent offers a number of unique design features that provide optimal luminal patency, precision, and ease of use.12 The hookcross design, which minimizes foreshortening and provides optimal radial and axial force so the stent expands enough to drain the bile duct lumen without causing undue pain or trauma to the surrounding tissue,12 makes a strong case for including the HANAROSTENT Biliary uncovered stent in the physician’s armamentarium for the management of malignant biliary obstruction.
“The unique features of the HANAROSTENT make it a good choice for many instances of malignant obstruction,” Dr Haber said. “It has a full range of sizes, allowing for bile ducts without completely straight axes, and enabling the physician to adapt the stent to the contours of the duct. It’s also a straightforward deployment, the said (Figure 2). “Most of the available stents have a sheath withdrawal aspect to releasing the stent, so the HANAROSTENT fits into that particular deployment modality, and I think it’s quite simple to learn.” Dr Heller added: “The HANAROSTENT is easy to deploy, and the use of radiopaque markers on the stent makes it user-friendly. It has a lot of potential clinical applications.”
References
- Jang S, Stevens T, Parsi M, et al. Association of covered metallic stents with cholecystitis and stent migration in malignant biliary stricture. Gastrointest Endosc. 2018;87(4):1061-1070.
- Kim SH, Lee DK, Kim HG, et al. Features of malignant biliary obstruction affecting the patency of metallic stents: a multicenter study. Gastrointest Endosc. 2002;55(3):359-365.
- Soderlund C, Under S. Covered metal versus plastic stents for malignant common bile duct stenosis: a prospective, randomized, controlled trial. Gastrointest Endosc. 2006; 63(7):986-995.
- lsayama H, Komatsu Y,、 Tsujino T, et al. A prospective randomized study of”covered”versus “uncovered” diamond stents for the management of distal malignant biliary obstruction. Gut. 2004;53(5):729-734.
- Lee JH, Krishna KG、 Singh A, et al. Comparison of the utility of covered metal stents versus uncovered metal stents in the management of malignant biliary stricture in 749 patients. Gastrointest Endosc. 2013, 78(2):312-324.
- Telford JJ, Carr-Locke DL, Baron TH, et al. A randomized controlled trial comparing uncovered and partially covered self-expandable metal stents in the palliation of distal malignant biliary obstruction. Gastroinrest Endosc. 2010;72(5):907-914.
- YoonWJ, Lee」K, Lee KH, et al. A comparison of covered and uncovered Wallstents for the management of distal malignant biliary obstruction. Gostrointest Endosc. 2006;63(7):996-1000.
- Nakai Y, lsayama H, Kogure H、 et al. Risk factors for covered metallic stent migration in patients with distal malignant biliary obstruction due to pancreatic cancer. JGastroenrerol Hepatol. 2014;29(9):1744-1749.
- Li J, LIT, Sun P, et al. Covered versus uncovered self-expandable metal stents for managing malignant distal biliary obstruction: a meta-analysis. PLoS One. 2016;11(2):eol 49066.
- lsayama H, Nakai Y, Hamada T, et al. Understanding the mechanical forces of self-expandable metal stents in the biliary ducts. Curr Gastroenterol Rep. 2016;18(12):64.
- Nakai, lsayama H, Kawakubo K, et al. Metallic stent with high axial force as a risk factor for cholecystitis in distal malignant biliary obstruction.
J Gascroenterol Heparol. 2014;29(7):1557-1562. - HANAROSTENT Biliary uncovered stent (product brochure). Center Valley, PA: Olympus America Inc; 2017. OAIET09178R023287.
- Soderlund C, Linder S、 Bergenzaun PE, et al. Nltinol versus steel partially covered self-expandable metal stent for malignant distal biliary obstruction: a randomized trial. Endoscopy. 2014;46(11):941·948.
Disclosures: Dr Haber is a consultant to and/or serves on the speakers bureau for Aries Pharmaceuticals, Inc; Boston Scientific; Cook Medical; Covidien; Endogastric Solutions, Inc; ERBE; Olympus America Inc; and Ovesco Endoscopy AG. Dr Heller has nothing to disclose.
Disclaimer: This article is designed to be a summary of information. While it Is detailed, It Is not an exhaustive clinical review. McMahon Publishing, Olympus, and the authors neither affirm nor deny the accuracy of the information contained herein. No liability will be assumed for the use of the article, and the absence of typographical errors is not guaranteed. Readers are strongly urged to consult any relevant primary literature.
Copyright©2018 McMahon Publishing, 545 West 45th Street, New York, NY 10036. Printed in the USA. All rights reserved, including the right of reproduction, in whole or in part, in any form.
- Keyword
- Content Type






