Improving Minimally Invasive BPH Treatment
iTind: Minimally Invasive Treatment for Relieving the Symptoms of Benign Prostatic Hyperplasia (BPH)
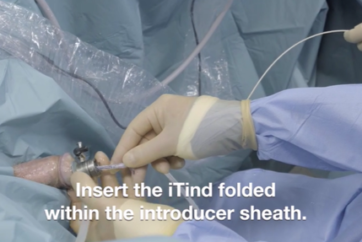
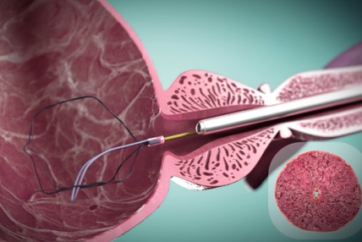
iTind is placed into the prostate in a folded configuration, where it expands and exerts localized ischemic pressure on the tissue. This pressure aims to reshape the tissue of the prostatic urethra and the bladder neck, creating three longitudinal channels through which urine can flow.
Patients are able to return home during the 5-7 day treatment, at the end of which the device is entirely removed, while the newly created channels aim to provide long-lasting1 relief of BPH symptoms.
Benefits

- BPH symptom relief of up to 3 years is clinically proven.
- Porpiglia F, Fiori C, Bertolo R et al. 3-Year follow-up of temporary implantable nitinol device implantation for the treatment of benign prostatic obstruction. BJU Int. 2018 Jul;122(1):106-112.
- Porpiglia F, Fiori C, Amparore D et al. Second-generation of temporary implantable nitinol device for the relief of lower urinary tract symptoms due to benign prostatic hyperplasia: results of a prospective, multicentre study at 1 year of follow-up. BJU Int. 2019; 123: 1061-1069.
- Kadner G, Valerio M et al. Second generation of temporary implantable nitinol device (iTind) in men with LUTS: 2 year results of the MT-02-study, World Journal of Urology, 2020 Mar.
- Content Type