Kei Morikawa, MD
Division of Respiratory and Infectious Diseases,
Department of Internal Medicine
St. Marianna University School of Medicine
Disclaimer:
- TXI™, RDI™, and NBI™ Technologies are not intended to replace histopathological sampling as a means of diagnosis
- The positions and statements made herein by Dr. Morikawa are based on Dr. Morikawa’s experiences, thoughts and opinions. As with any product, results may vary, and the techniques, instruments, and settings can vary from facility to facility. The content hereof should not be considered as a substitute for carefully reading all applicable labeling, including the Instructions for Use. Please thoroughly review the relevant user manual(s) for instructions, risks, warnings, and cautions. Techniques, instruments, and setting can vary from facility to facility. It is the clinician’s decision and responsibility in each clinical situation to decide which products, modes, medications, applications, and settings to use.
- TXI™, RDI™, and NBI™ Technologies are 510(k) cleared in the United States. The case study is being furnished to provide an example of this technology use. The BF-1TH1200 used in this case is not available in the US market at this time nor is there an established time for its release. The safety and effectiveness of this product and or the use of these products has not yet been established in the United States market.
- The EVIS X1™ endoscopy system is not designed for cardiac applications. Other combinations of equipment may cause ventricular fibrillation or seriously affect the cardiac function of the patient. Improper use of endoscopes may result in patient injury, infection, bleeding, and/or perforation. Complete indications, contraindications, warnings, and cautions are available in the Instructions for Use (IFU)
- Dr Morikawa, the authoring physician(s) of this presentation, are/ is a paid consultant(s) to Olympus Corporation
Scope: BF-1TH1200
Location: Upper part of the trachea
Patient information: Male, 78 years old
Medical history: Intubation and tracheostomy were performed 2 years ago due to COVID-19 pneumonia. Chemotherapy was administered to treat mantle cell lymphoma 1 year ago. After complete remission of the lymphoma, PET-CT indicated a suspicion of a tumorous lesion on the right tracheal wall. He was referred to our institution for further examination. He is an ex-smoker who stopped smoking 3 years ago with a history of 27.5 pack-years.
Case Video
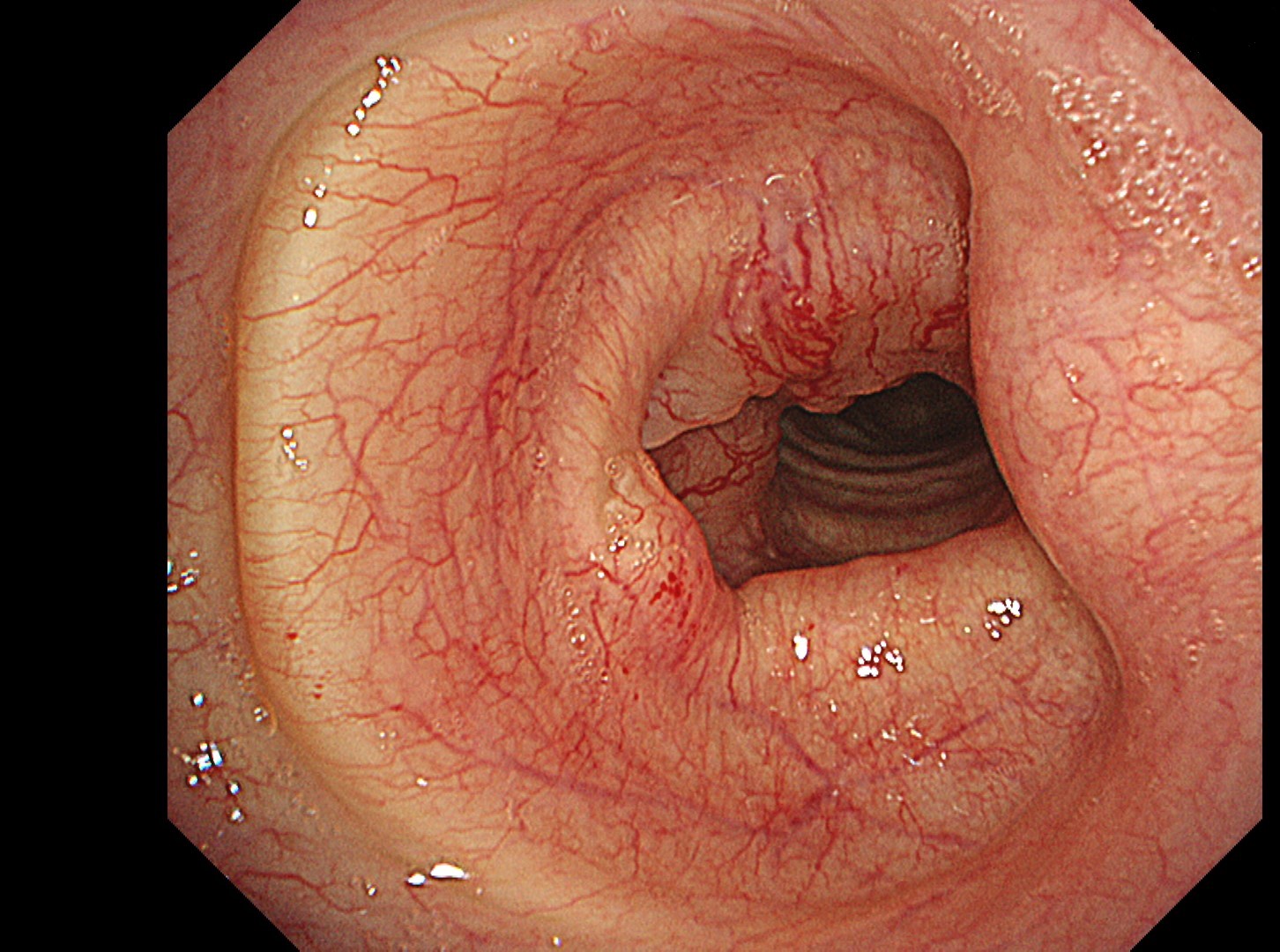
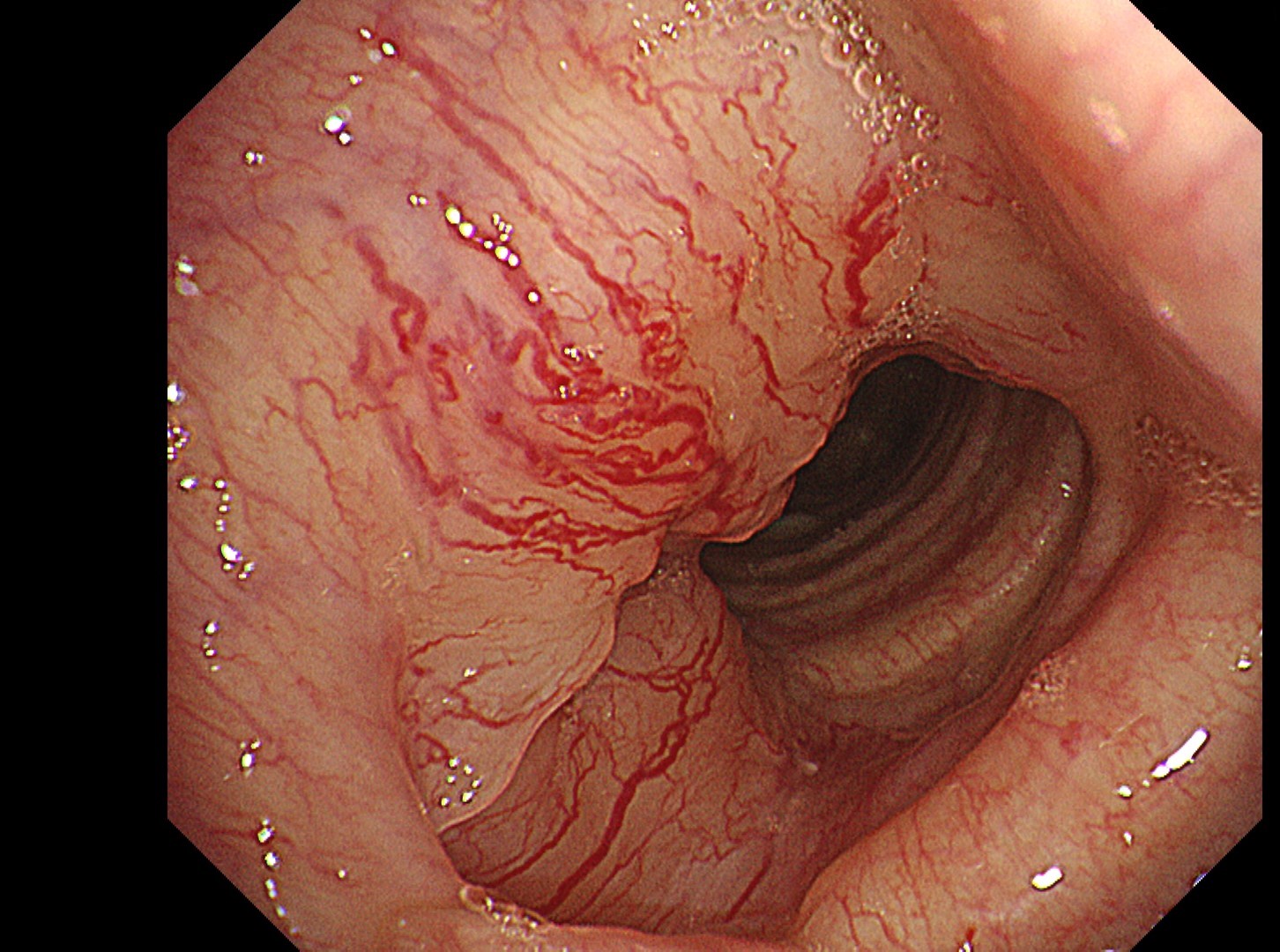
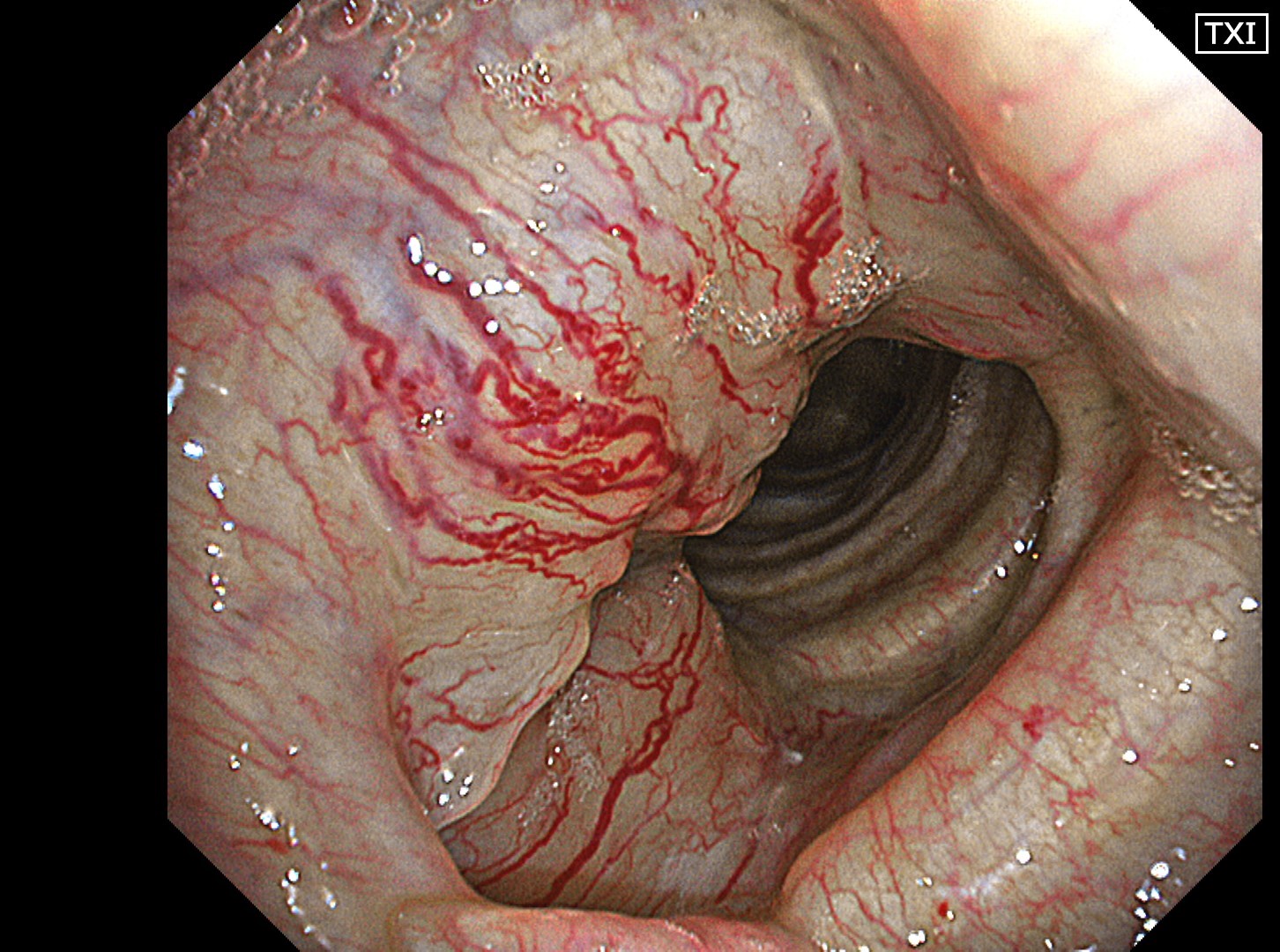
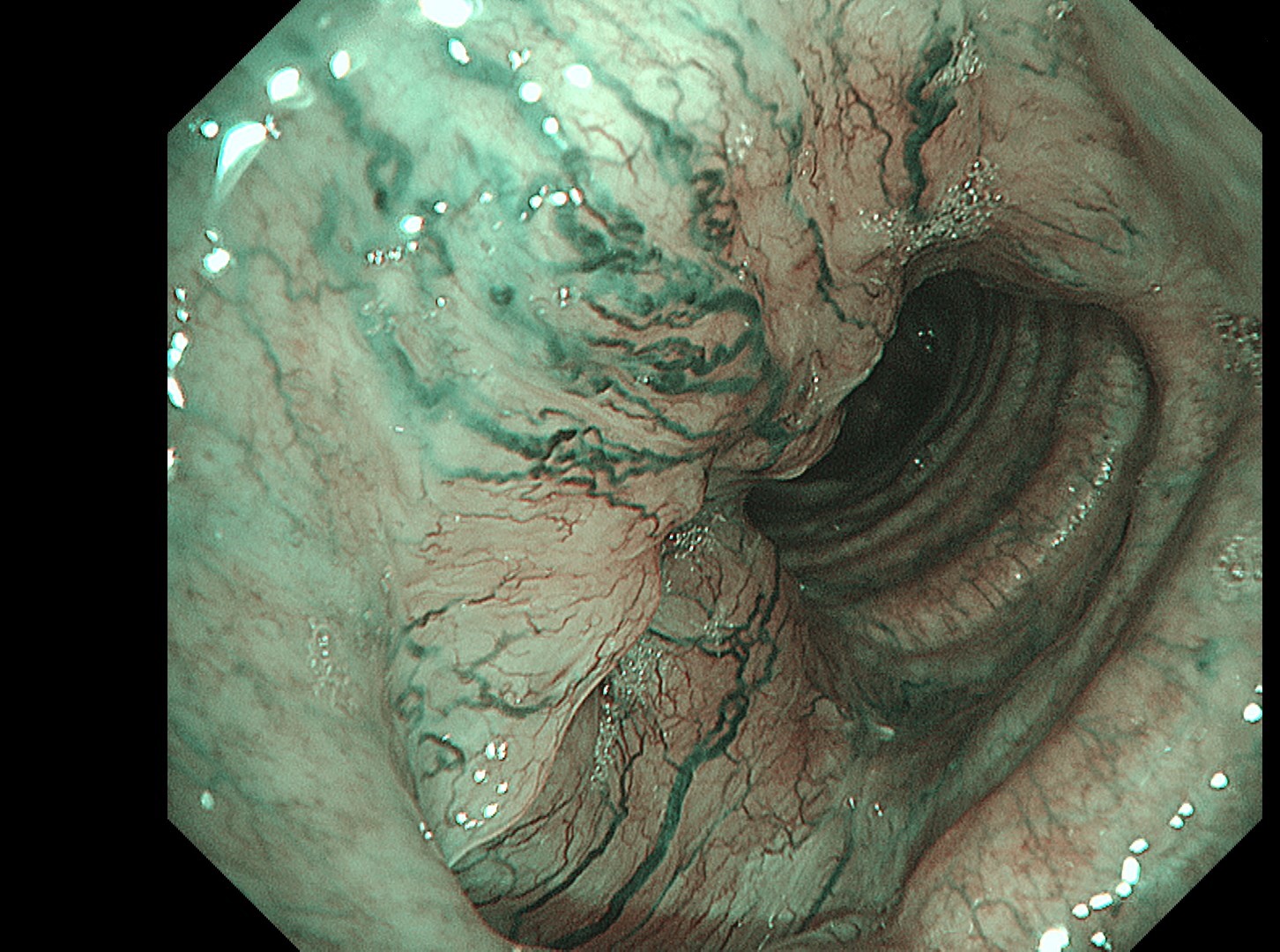
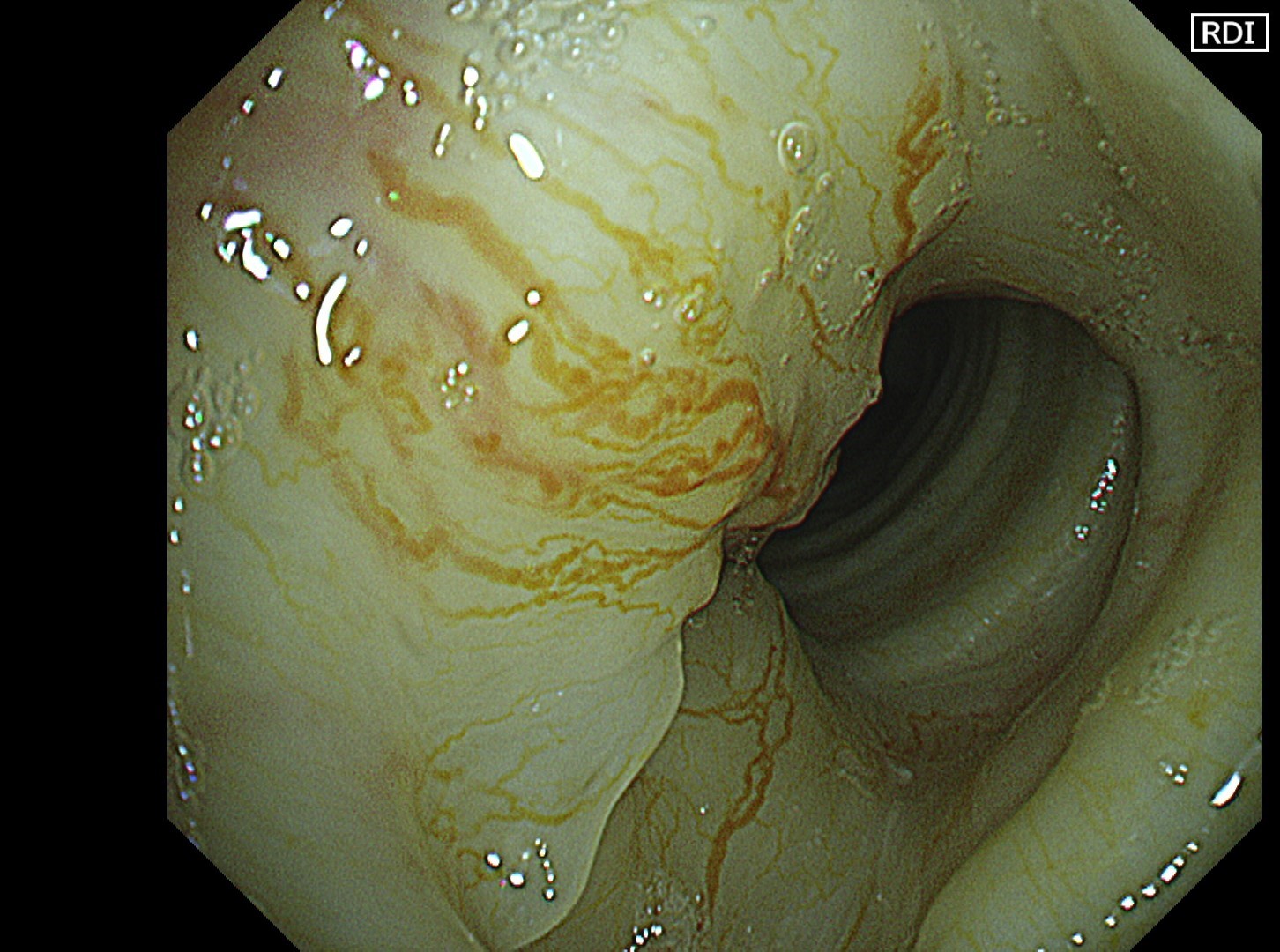
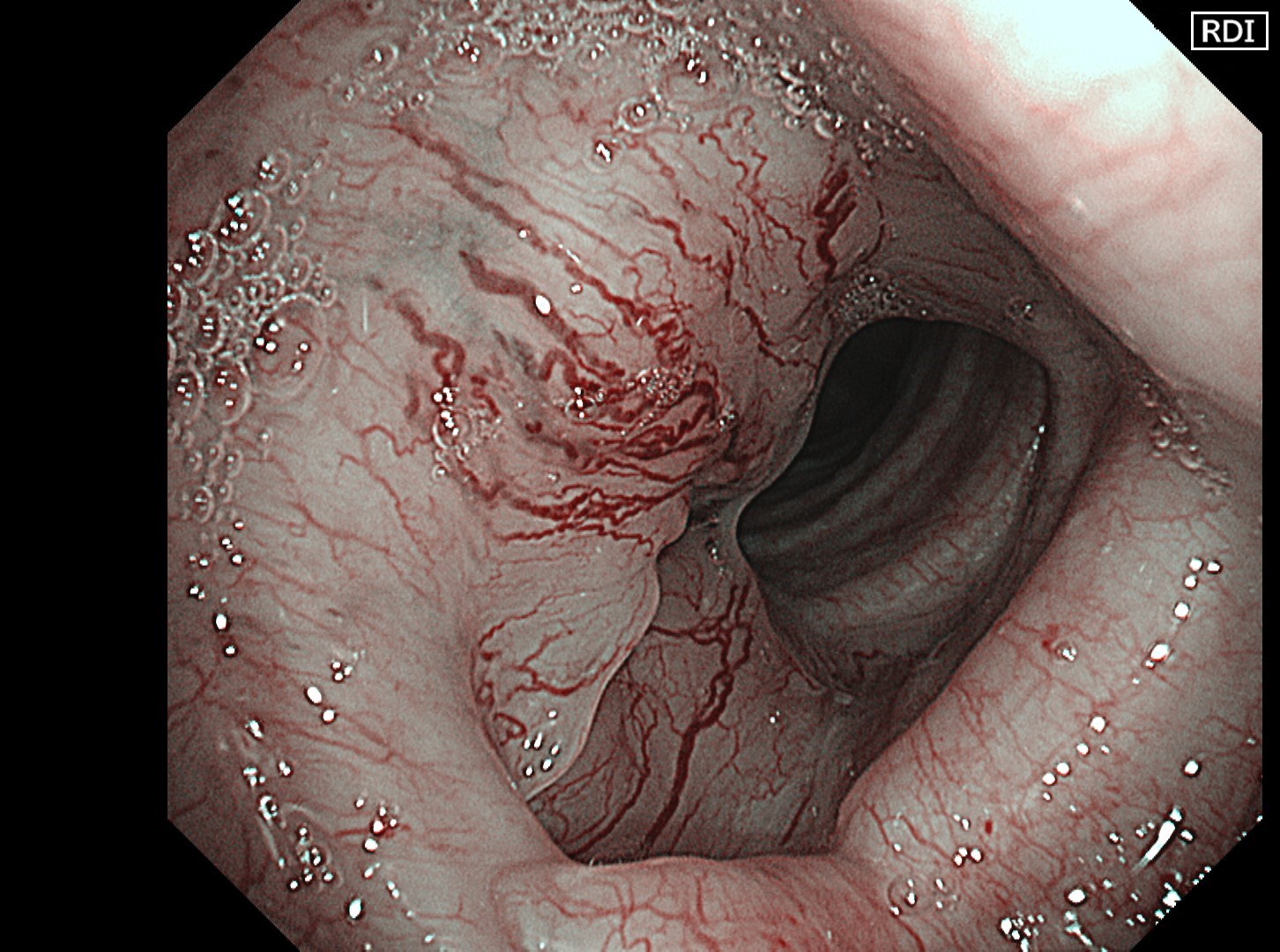
The flat tumor growth in the upper part of the trachea shows a distribution pattern of subepithelial vessels suspected to be malignant and is carefully observed in detail in the NBI™, TXI™, and RDI™ technologies modes. The lesion is subsequently biopsied while taking extra care not to cause bleeding.
Pathological Findings
- Figure A (HE staining): Tumor foci composed of proliferation of ductal epithelial cells and myoepithelial cells are observed. These feature a mesh-like structure including small cystic parts.
- Figure B (p63 immunostaining): p63-positive myoepithelial cells are observed at the periphery of the tumor foci.
- Final diagnosis: Adenoid cystic carcinoma


Overall Comment
In this case, abnormal subepithelial vessels that had increased irregularly were observed in detail in the TXI, NBI, and RDI modes. Since the lesion was located below the glottis, evaluation of the vessels in each mode was considered useful for careful selection of a biopsy site to prevent bleeding. The result suggests that the TXI™ and RDI™ technology 3 settings, in particular, have the potential to be equivalent or superior to NBI™ technology for observation of vessels.
Co-editor:
Dr. Nobuyuki Ohike
Department of Pathology, St. Marianna University School of Medicine
- Content Type