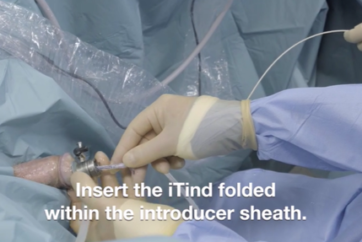
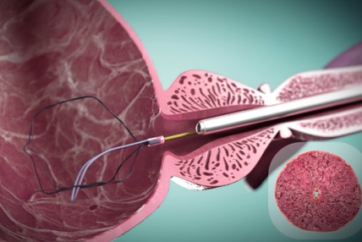
Clinical Evidence on iTind: Explore the Latest Research Findings
Discover the latest and most relevant clinical evidence on iTind, a minimally invasive treatment for Benign Prostatic Hyperplasia (BPH), through our curated list of research findings. This page serves as a resource for researchers, clinicians, and healthcare professionals interested in staying informed about the efficacy and impact of iTind in relieving the symptoms of BPH. Each entry includes a link to the abstract for further reading.
Clinical Evidence on BPH
Trifecta outcomes to assess the feasibility of local anesthesia for benign prostate hyperplasia minimally invasive surgical treatments
| Author | Olivero A, Khadhouri S, Palagonia E, et al. |
| Publication Year | 2025 |
| Journal |
Urology Annals |
One-Year Outcomes of a UK Center Delivering Minimally Invasive Surgery for Bladder Outflow Obstruction Using Local Anesthetic Without Sedation in the Office Setting
| Author | Khadhouri S, Turnbull M, Drummond L, et al. |
| Publication Year | 2025 |
| Journal |
Journal of Endourology |
First Multi-Center, Real-World Study on the Temporary Implantable Nitinol Device (iTIND) for the Management of Lower Urinary Tract Symptoms Related to Benign Prostatic Obstruction.
| Author | Castellucci R, Secco S, Olivero A, et al. |
| Publication Year | 2025 |
| Journal |
Société Internationale d’Urologie Journal |
I-Tind for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: Mid-term outcomes from a multicenter cohort
| Author | Dimitri M, Calarco A, Filippi B, et al. |
| Publication Year |
2025 |
| Journal |
Urologia |
Temporary implantable nitinol device for benign prostatic hyperplasia-related lower urinary tract symptoms: over 48-month results
| Author | Amparore D, De Cillis S, Schulman C, Kadner G, Fiori C, Porpiglia F. |
| Publication Year | 2023 |
| Journal |
Minerva Urology and Nephrology |
Temporarily implanted nitinol device versus prostatic urethral lift for minimally invasive surgical treatment of benign prostatic hyperplasia with lower urinary tract symptoms: a matching-adjusted indirect comparison
| Author | Kernen KM, Omar S, Goodnight B, Skodny P, Bruce S, Yu TM. |
| Publication Year | 2023 |
| Journal |
The Canadian Journal of Urology |
3-Year results following treatment with the second generation of the temporary implantable nitinol device in men with LUTS secondary to benign prostatic obstruction
| Author | Amparore D, Fiori C, Valerio M, et al. |
| Publication Year | 2021 |
| Journal |
Prostate Cancer and Prostatic Diseases |
Urinary and sexual function after treatment with temporary implantable nitinol device (iTind) in men with LUTS: 6-month interim results of the MT-06-study
| Author | De Nunzio C, Cantiello F, Fiori C, et al. |
| Publication Year | 2021 |
| Journal |
World Journal of Urology |
The iTind Temporarily Implanted Nitinol Device for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: A Multicenter, Randomized, Controlled Trial
| Author | Chughtai B, Elterman D, Shore N, et al. |
| Publication Year | 2020 |
| Journal |
Urology |
Second generation of temporary implantable nitinol device (iTind) in men with LUTS: 2 year results of the MT-02-study
| Author | Kadner G, Valerio M, Giannakis I, et al. |
| Publication Year | 2020 |
| Journal |
World Journal of Urology |
Second-generation of temporary implantable nitinol device for the relief of lower urinary tract symptoms due to benign prostatic hyperplasia: results of a prospective, multicentre study at 1 year of follow-up
| Author | Porpiglia F, Fiori C, Amparore D, et al. |
| Publication Year | 2019 |
| Journal |
BJU International |
How I Do It: Temporarily Implanted Nitinol Device (iTind)
| Author | Elterman D, Gao B, Zorn KC, Bhojani N, Chughtai B |
| Publication Year | 2021 |
| Journal |
Can J Urol. |
* The information on this page was last updated on November 18, 2024.
- Content Type