Douglas G. Adler MD, FACG, AGAF, FASGE1
Professor of Medicine | Director, GI Fellowship Program
Gastroenterology and Hepatology | University of Utah School of Medicine Huntsman Cancer Center
Disclosures: Douglas G. Adler, MD: Consultant to Olympus
Introduction
Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is a long-established and highly proven endoscopic technique that is widely used to obtain tissue and fluid samples from solid and cystic lesions within or abutting the luminal gastrointestinal tract. Many EUS-FNA needles are currently commercially on the market and available for clinical use, and studies of these needles have shown similar efficacy in terms of tissue acquisition and sample adequacy.
In recent years attention has turned to the development and clinical implementation of EUS Fine Needle Biopsy (FNB) needles, also referred to as “core needles” or “core biopsy needles” as these devices are designed to obtain larger tissue samples that can undergo histologic analysis, and not simply cytologic analysis. Core samples can also undergo specialized testing to allow for personalized medicine in oncology patients. There is ongoing research and development into new EUS-FNB needles.
This white paper will detail a single operator evaluation and experience with a new EUS-FNB needle (EZ Shot 3 Plus, Olympus, Center Valley, PA). This white paper will evaluate clinical and pathologic outcomes when using the EZ Shot 3 Plus device to sample solid pancreatic lesions.
Methods
The device under evaluation in this study is the EZ Shot 3 Plus FNB needle. The device operates in a standard fashion, has a needle made of nitinol alloy*, a sheath made of a multi-layered metal coil, and utilizes a Menghini needle tip configuration.
The first ten patients with solid pancreatic lesions to undergo EUS-FNB with the EZ Shot 3 Plus device were studied. EUS procedures including FNB were performed during November 2020. Patients were included if they were >18 years of age and had a solid pancreatic lesion identified on cross sectional imaging that was felt to be clinically significant and warranted endoscopic tissue acquisition. Patients were excluded if they were <18 years of age, pregnant, prisoners, or had imaging abnormalities warranting biopsy other than solid pancreatic lesions.
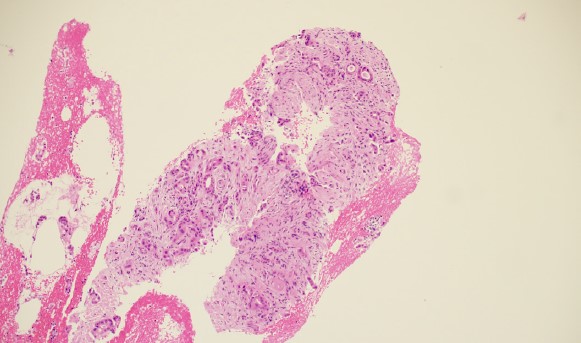
Data analyzed included patient demographics, the size and location in the pancreas of the target lesion, the ease of needle operation, the size of the target lesion, ease of tissue puncture, the route of needle passage (i.e. transgastric or transduodenal), the number of needle passes obtained per patient, the echogenicity of the needle tip, the FNB technique utilized (i.e. slow pull, fanning, wet suction, dry suction, etc), whether or not a core of tissue was obtained, how the tissue was analyzed (cytology, histology, or both), tissue adequacy, the final diagnosis reached in each patient, whether or not the patient had to undergo a repeat procedure to obtain additional tissue, and the presence and severity of any adverse events incurred during EUS-FNB. Needle tip echogenicity was graded on a scale of 1 to 4, with 1 being the most echogenic and 4 being the least echogenic.
In all cases, rapid onsite evaluation (ROSE) by a board certified cytopathologist was performed. The cytologist had the option of placing all tissue into formalin for later histologic evaluation or placing some of the tissue obtained into formalin and using some of the tissue for immediate staining and cytologic evaluation. Tissue acquisition was felt to be adequate if the cytologist was able render a final diagnosis based on the supplied tissue. The presence or absence of a true tissue core was assessed macroscopically and microscopically by the presence of a solid tissue core (excluding blood clots) when evaluated both during the EUS procedure and at a later time after formal processing by pathology. This analysis was IRB approved.
Results
Demographics
Over the study period, 10 consecutive patients (6M, 4F) with a mean age of 69.6 years underwent EUS-FNB of solid pancreatic lesions using the EZ Shot 3 Plus EUS core needle device. The mean lesion size was 31.5mm in greatest cross sectional diameter. Four lesions were located in the pancreatic head, 1 was in the uncinate process, 1 was in the genu, 2 were in the body and 2 were in the tail. Nine of the 10 patients had not undergone prior EUS-guided tissue sampling. One patient had undergone a prior EUS-FNA with a different needle device that was non-diagnostic and the patient was referred for a second attempt.
Needle Use Details
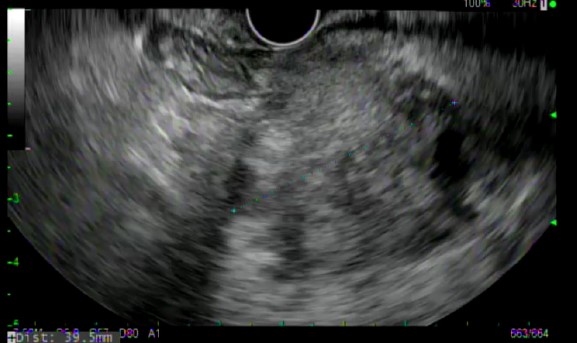
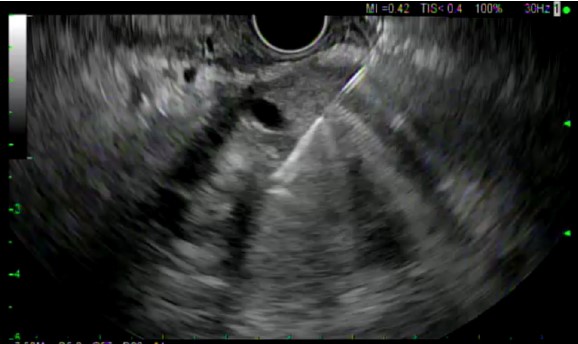
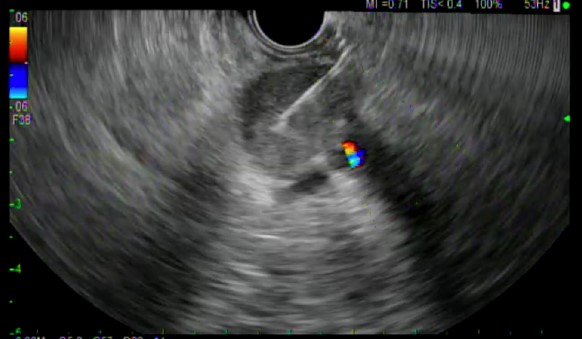
All ten patients underwent EUS-FNB with a 22 gauge EZ-Shot 3 Plus needle. (Figures 1 and 2) Five out of 10 patients underwent biopsy via the transgastric route and the other 5 underwent biopsy via the transduodenal route. The number of needle passes obtained from each patient ranged from 1-4, with a mean of 1.9 needle passes per patient. Four patients required only 1 needle pass, four patients required 2 passes and one patient each required 3 and 4 passes, respectively.
At least one visible tissue core was obtained in 10/10 patients. The Slow-Pull technique (whereby the stylet was slowly withdrawn a distance of 6-12 inches during needle actuations) was used in all patients, and 2/10 patients underwent Fanning during needle actuations. The ability of the needle to puncture the target tissue was felt to be excellent in all cases, as were needle operation and echogenicity. Echogenicity was graded at a level 1 in all cases.
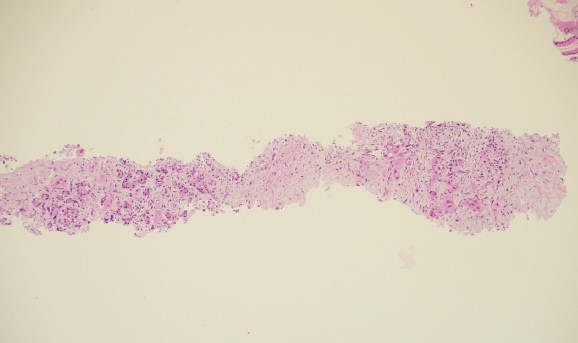
Tissue Analysis
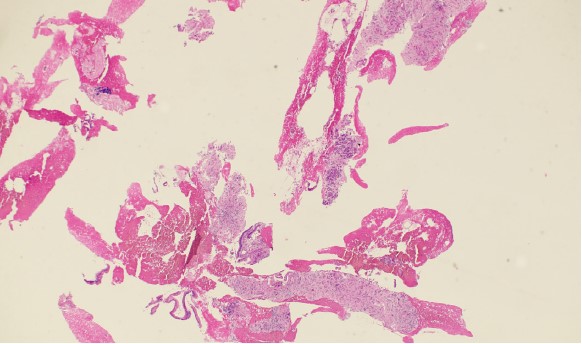
The cytopathologists elected, in all cases, to split the tissue core samples with the majority of tissue being placed into formalin for later histologic evaluation. A small amount of tissue was taken and used to perform Rapid Onsite Cytologic Evaluation (ROSE) by a board certified cytopathologist, typically via “smash prep” (whereby a small section of a tissue core is compressed between two glass slides and smeared prior to staining). The tissue used for cytologic evaluation was processed in the endoscopy lab and stained via Hematoxylin and Eosin (H&E) staining in real time during the endoscopic ultrasound procedure. ROSE tissue sampling was felt to be adequate in 10/10 patients. No patient required a repeat biopsy at a later date.
In 9/10 patients, the final cytologic and histologic diagnoses were pancreatic adenocarcinoma. One patient with a 24mm solid pancreatic tall mass had a final cytologic and histologic diagnosis of accessory spleen tissue (splenosis).
Adverse Events
There were no immediate or delayed adverse events in any patient.
Discussion
This pilot study shows a high degree of diagnostic accuracy when using the EZ Shot 3 Plus EUS FNB needle. Tissue cores were produced in all patients. The tissue obtained was used for both cytologic and histologic evaluation in all patients, although if ROSE were not available (as is the case at some centers) the tissue cores could have simply been placed into formalin in their entirety for later histologic evaluation.
The overall efficacy of the needle was felt to be high. Fully 40% of patients only required one needle pass to obtain adequate tissue for a formal diagnosis. In the patients who required more than 1 needle pass, it should be stressed that the additional passes were to obtain more tissue for ROSE but that in all of these patients the tissue cores were themselves ultimately felt to be diagnostic. The ease of operation and ultrasound visibility of the needle were felt to be high in all cases. There were no adverse events seen in this study.
All patients were able to receive a final cytologic and histologic diagnosis in a single procedure, including one patient who had undergone prior EUS-guided tissue sampling without receiving a final tissue diagnosis. It is not known what type of needle this patient’s prior tissue sampling was performed with as the procedure occurred at an outside hospital and the clinical notes did not specify the needle brand, type, or size selected.
The strengths of this study include a single operator with extensive experience in all facets of EUS and widespread experience with EUS-FNA and FNB devices. The focus on solid pancreatic masses, perhaps the most common indication for EUS-FNA, increases the applicability of these results to a broad range of endosonographers. Future studies of this device should be larger, multicenter, and focus on additional target lesion types beyond solid pancreatic masses.
Conclusion
The EZ Shot 3 Plus EUS-FNB needle had a high degree of efficacy and safety in this pilot study.
- Keyword
- Content Type