PneumoLiner
Containment Device
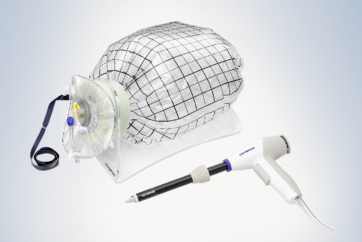
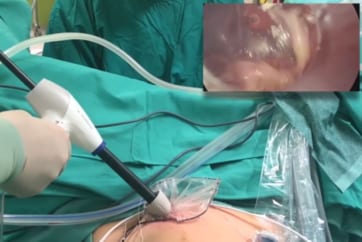
PneumoLiner is the first and only power morcellation containment device specifically designed for intra-abdominal insufflation during GYN procedures.

The PneumoLiner is uniquely designed to:
- Conform to each patient’s abdominal size, avoiding intra-abdominal folding of material which can restrict movement, visualization, or inadvertent capture by the morcellator.
- Provide a barrier between target tissue and non-targeted abdominal contents.
- Maintain a barrier to the escape of fluids, cells and tissue fragments.
FDA Clearance & Letters:
WARNING:
Information regarding the potential risks of a procedure with this device should be shared with patients. Uterine tissue may contain unsuspected cancer. The use of laparoscopic power morcellators during fibroid surgery may spread cancer. The use of this containment system has not been clinically demonstrated to reduce this risk.
CONTRAINDICATIONS: Do not use on tissue that is known or suspected to contain malignancy. Do not use for removal of uterine tissue containing suspected fibroids in patients who are: peri- or post-menopausal; or candidates for en bloc tissue removal, for example, through the vagina or via a mini-laparotomy incision. Do not use in women with undiagnosed uterine bleeding. Do not use this device on patients with known or suspected allergies to polyurethane. Do not use where the abdominal wall thickness is larger than 10cm. This device should only be used by physicians who have completed the formal validated training program administered by Olympus and/or Advanced Surgical Concepts.
For more information, please read the full PneumoLiner Instructions for Use for indications, additional contraindications, warnings and precautions.
For additional information, please view the videos below from AAGL 2015.
- Content Type