Real-World Insights using Thulium Fiber Laser
This Clinical Case Atlas highlights physician experiences using Olympus’ Soltive™ laser technology for treatment of urolithiasis and BPH (Benign Prostatic Hyperplasia).
Through the following series of case studies, you will learn how various clinicians approach challenging procedures, optimize their instrument handling, and leverage laser solutions to achieve desired patient outcomes.
Ureteroscopy Case Studies
Case 1: An Impacted Ureteral Stone
Dr. Mitchell Humphreys’s Strategy
Case Study 1:
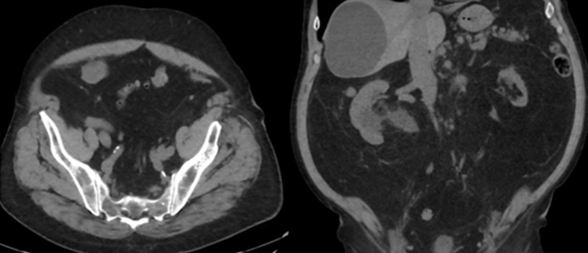
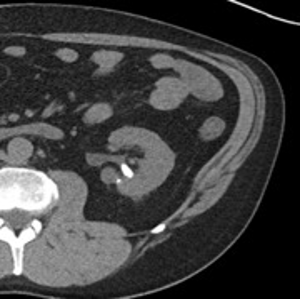
74-year-old male with a past medical history of nephrolithiasis, sleep apnea, thoracic aortic aneurysm, activated protein C resistance, Factor V deficiency, Gout, Hypertension and benign prostatic hyperplasia (BPH) presented for further evaluation regarding nephrolithiasis. The patient recently underwent extracorporeal shock wave lithotripsy (EWSL), and a post operative abdominal x-ray showed a calcification at the level of the right sacrum and a right ureteral stone could not be ruled out. A non-contrast CT abdomen and pelvis was ordered, which showed severe right hydroureteronephrosis due to two stacked distal right ureteral stones measuring 11 mm in size (Figure 3). Hounsfield units of the ureteral stones averaged 1895.
The patient underwent a right ureteroscopy and laser lithotripsy. Using a semi-rigid ureteroscope, a large impacted and obstructing stone was encountered. A hydrophilic wire was advanced past the stone into the renal pelvis. A ureteral catheter was advanced over the hydrophilic wire into the renal pelvis under fluoroscopic guidance, and the hydrophilic wire was exchanged for a super-stiff wire. Due to edema and tortuosity of the ureter, the semi rigid ureteroscope could not safely maintain visualization of the stone. Therefore, a 12/14 Fr x 28 cm ureteral access sheath was advanced to the level of the stone and a flexible ureteroscope was advanced to the level of the stone, to allow optimal visualization and laser lithotripsy.
A 200μm thulium laser fiber was advanced through the ureteroscope in conjunction with the TFL (Soltive™ SuperPulsed Laser System, Olympus) platform. Low energy settings (1J, 2 Hz) were used with short pulse, and fragmentation technique to minimize the risk of ureteral injury and reduce stone retropulsion. Pauses every 30 seconds were taken during the procedure to avoid thermal injury to the ureter. Stone fragments were successfully retrieved using a basket. After all stone fragments were extracted, there was no evidence of ureteral injury or residual stones.
The kidney stone analysis identified the stone type as 70% calcium oxalate monohydrate and 30% calcium phosphate. We utilized these low energy settings for this specific stone as it was highly impacted. In these cases, we attempt to use lower energy settings in the ureter (a confined space) to minimize risk of thermal injury.
A post operative CT scan showed no residual ureteral stone and resolution of the acute hydronephrosis (Figure 4). Additionally, a nuclear medicine renal Lasix scan was performed to assess for distal right ureteral obstruction and no obstruction was noted.
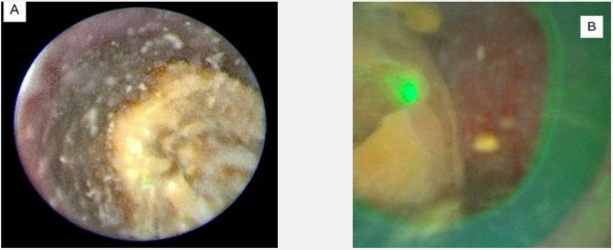

Case 2: Soltive™ TFL Versatility for Ureteral Stones
Dr. Brian Orr’s Strategy
Placement of a ureteral access sheath can certainly aid in the ability in treating renal stones, which is especially true for surgeons that use a fragmentation and basket approach to stone extraction. Without an access sheath, fragmentation and stone extraction can be incredibly inefficient and sometimes impossible for renal stones.
In clinical settings where an access sheath can be placed atraumatically, fragmenting or dusting can ensue without restriction per the surgeon’s preference. When an access sheath cannot be placed due to a tight ureter or other anatomic limitations, the Soltive™ TFL can be utilized for dusting, potentially eliminating the need for a staged secondary ureteroscopy.
A smaller ureteroscope can be navigated adjacent to or backloaded over a wire to provide efficient, safe treatment of kidney stones without the need for sheath placement.
Case Study 2:
A 47-year-old woman presents to the Emergency Department for flank pain. CT scan shows a 10 mm stone in the proximal left ureter with hydronephrosis, and the urinalysis confirms no evidence of infection. The patient desires a ureteroscopy with the goal to render her stone free within a single procedure. In this case scenario, the likelihood of being stone free in a single procedure will largely depend on an anatomically favorable ureter.
If this patient’s ureter is very accommodating, a ureteral access sheath can be passed up to the stone and fragmenting or dusting can ensue per the surgeon’s preference. Similarly, if desired, a rigid ureteroscope may be passed up to the stone and fragmentation or dusting can be performed. However, commonly when neither an access sheath nor rigid scope can be passed, this leads to simply placing a stent and staging a secondary ureteroscopy 1-2 weeks later. This outcome is frustrating for both the patient and the surgeon.
In my practice, I prefer using a flexible ureteroscope, passing it next to the wire, and simply dusting he stone with the Soltive™ System, this is often completed within 5-10 minutes; all while minimizing risk of undue trauma to the ureter with an access sheath. Flexible ureteroscopes are generally smaller in diameter than rigid scopes and thus can navigate up troublesome ureters more successfully, particularly for proximal stones. In my experience, a 1-french difference in diameter between scopes can dictate success in navigating a tight ureter.
In this case, neither an access sheath nor a rigid ureteroscope could be safely passed, so I therefore chose a flexible ureteroscope (any size or SU/RU) to be used in a sheathless fashion, passing it alongside the wire. Using a 150 micron laser fiber, I used settings of 0.2J at 60 Hz (left pedal) and 0.6J at 20 Hz (right pedal) for a total of 12W of power. Once the stone was dusted sufficiently, the final fragment was grabbed with a basket and pulled out and sent for chemical analysis, demonstrating a composition of 90% calcium oxalate monohydrate and 10% calcium phosphate.
Passing a flexible ureteroscope in a sheathless fashion using stone dusting with the Soltive System as was done in this case saved this patient a second, staged procedure. An access sheath could not be safely placed and a rigid ureteroscope could not be passed to the proximal ureter, yet the Soltive System allowed for quick, safe and effective dusting to be done on the first attempt using flexible ureteroscopy.
Overall, having a laser that can quickly and efficiently dust stones, without the need for an access sheath or dependence on a rigid scope, radically improves stone free rates on the first surgical attempt in patients with less accommodating ureters.
Treating Renal Stones Case Studies
Treating Large Renal Stones: SOLTIVE™ Thulium Laser
Dr. Lipkin’s Strategy using TFL laser lithotripsy
Improvements in technology have facilitated the treatment of larger and more complex intrarenal stones using flexible ureteroscopy with laser lithotripsy. Stone dusting as a technique has been shown to be effective at efficiently treating intrarenal stones. Improvements in laser technology have greatly facilitated this evolution in stone treatment. The thulium fiber laser (TFL) in particular, with its low peak power, is especially effective at stone dusting.
To treat larger intrarenal stones when performing ureteroscopy, ≥ 1.5 cm, consideration should be made to use a ureteral access sheath to allow for continuous irrigation. Irrigation is important to maintain visibility and minimize the potential impact of heat generation from the laser. If an access sheath is not able to be advanced up the ureter, a flexible ureteroscope can be inserted directly into the kidney to perform laser lithotripsy. In these cases, it can be helpful to leave a catheter in the bladder to facilitate drainage and flow.
Once the stone is identified, it is ideal to displace it into an upper or interpolar calyx to facilitate laser lithotripsy and to allow for passage of dust. It can also be helpful to place the patient in slight Trendelenburg to keep the stone in the upper pole location. The choice of laser fiber size is at the discretion of the surgeon, but typically 150 μ or 200 μ are used. Typically, I use the 200 μ fiber. The 150 μ is helpful when maximal deflection is needed to treat lower pole stones.
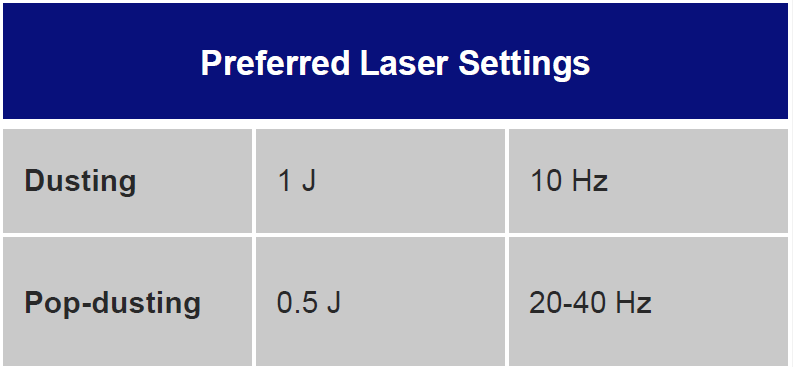
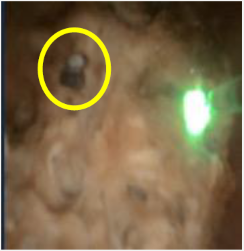
Laser settings should be tailored to the renal stone and technique. Based on benchtop data, for dusting I use 1 J and 10 Hz as studies indicate higher energy leads to greater stone ablation (Figure 1). Alternatively, 0.5 J and 20 Hz or 0.2J and 50 Hz can be used. Total energy should be kept at ≤ 20 Watts to avoid excessive heat generation. Sparks can be encountered (Figure 2), especially for calcium phosphate stones, which can lead to charring (Figure 3).
Charring can dramatically reduce dusting efficiency. Benchtop studies indicate low energy/high frequency settings are more likely to create char. If char is encountered, frequency should be reduced, and energy can be increased.
The stone should be systematically dusted, typically working around the edges (Figures 4-7). As the stone gets smaller, or if there are several smaller fragments at the end, pop-dusting can be performed. Preferred settings are in Figure 1. Adequate irrigation is important to avoid heat generation during pop-dusting. The goal is to create dust that is similar to appearance in Figure 8. Care should be taken to flush the dust and identify any residual fragments below the dust.
The patient can be taken out of Trendelenburg to allow for flushing of dust out of the kidney. A basket can also be used to grasp in the dust to identify any residual fragments that require further treatment. At the end of the procedure placement of a ureteral stent is left to the discretion of the surgeon.

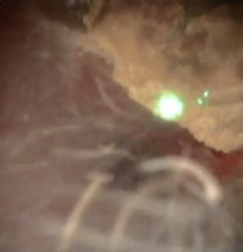
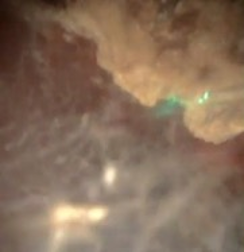

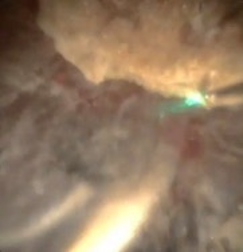
Treating Large Renal Stones: Soltive™ Thulium Laser (continued)
Dr. Lipkin’s Strategy of TFL laser lithotripsy
Case Study
Patient is a 60-year-old male with a 1.5 cm left intrarenal stone found on CT (Figure 9). He was having left flank pain and gross hematuria, and a decision was made to treat his stone. We discussed surgical options including percutaneous nephrolithotomy, shock wave lithotripsy and ureteroscopy with laser lithotripsy.
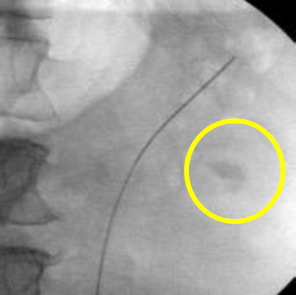
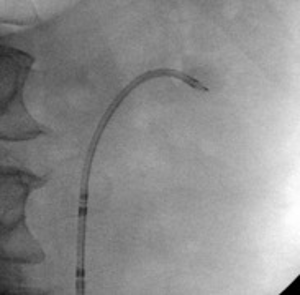
A decision was made to proceed with ureteroscopy. A pre-operative urine culture was obtained to rule out infection. Scout fluoroscopic images demonstrated the stone in an interpolar calyx (Figure 10). A flexible ureteroscope was used in this case without an access sheath. The scope was advanced over the wire into the kidney.
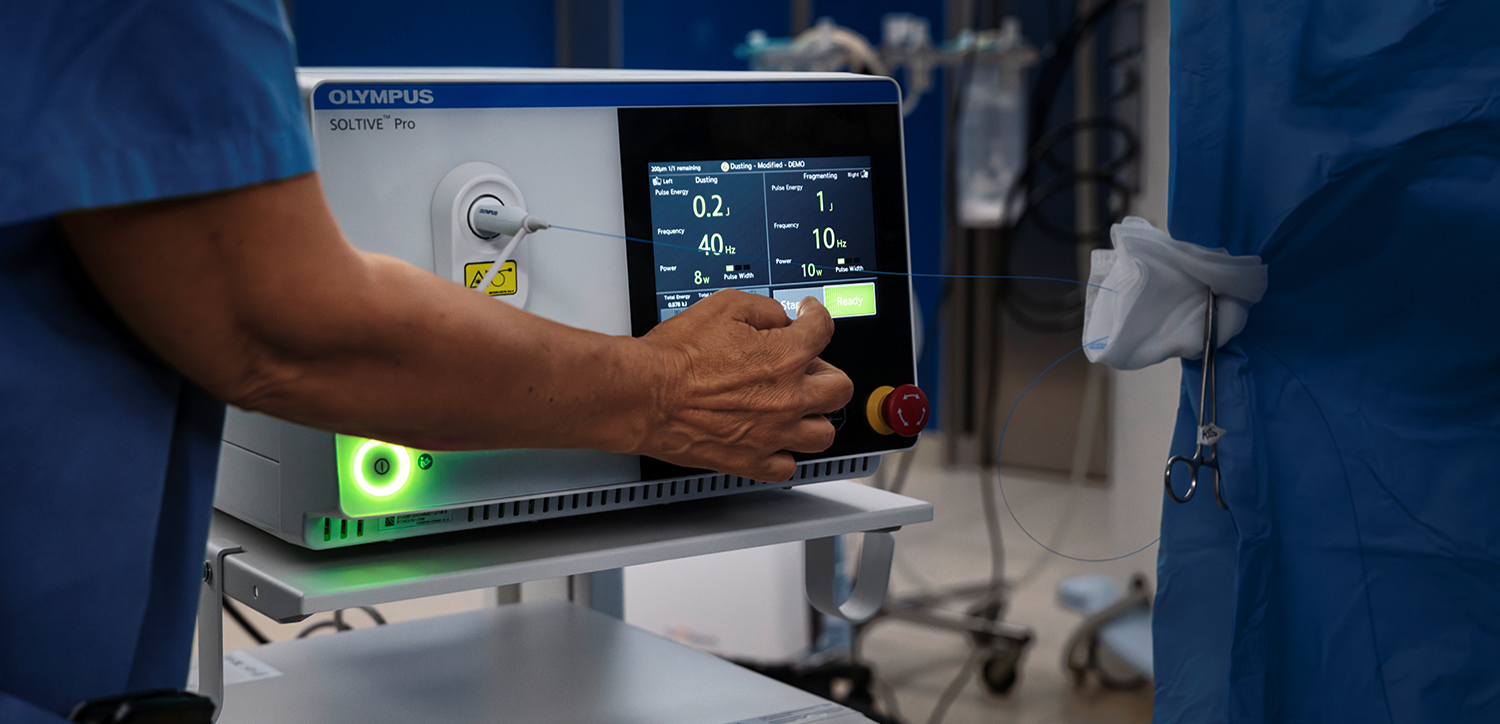
The stone was dusted using TFL at settings of 1 J and 10 Hz. Figure 11 shows partway through dusting and Figure 12 shows after dusting was completed. 0.5 J and 20 Hz to pop dust which is routine. The remainder of the kidney was inspected, and no residual fragments were noted. A wire was replaced into the kidney. The ureter was visually inspected as the ureteroscope was withdrawn. A ureteral stent was placed at the end of the procedure and left in place for 5 days.
Treating Large Renal Stones: Soltive™ Thilium Fiber Laser
Dr. Orr’s Strategy
Management of large renal stones will depend on the skillset and preferences of the surgeon, stone characteristics (size, location, number of stones, stone density) and patient comorbid conditions (anticoagulation status and other notable medical conditions).
PCNL, mini-PCNL, ultra-mini PCNL, ureteroscopy, staged ureteroscopy, CVAC and ESWL are all options for renal stones, but the best choice depends on a combination of many of the factors mentioned above. The Soltive Thulium Fiber Laser (TFL) has radically improved the number of stones that can be treated with a single ureteroscopic procedure.
The following are my recommendations for different types of large renal stones.
Large Renal Pelvic Stones:
Can be treated like any calyceal stones with the same laser settings. The only difference is fragmentation of the stone does not have
much of a “vacuum” effect on fragments into the laser fiber as is seen when the stones are calyceal. This is the reason for my
recommendation of moving the stone, if possible, into a calyx as the efficiency of dusting drastically increased when the stone has less
area to move around.
Large upper/midpole calyceal stones treated with Ureteroscopy:
Large upper and midpole calyceal stones tend to be much easier to treat than lower pole stones due to deflection requirements to access
lower pole stones. If the large stone is in a midpole or upper pole calyx, stone fragmentation or dusting can ensue immediately, generally
without the need to move or manipulate the stone to a different calyx. For a large, solitary, midpole stone, my preferred approach is to use
settings of 0.6 J and 30 Hz which will first provide fragmentation into smaller stone fragments around 5-10 mm.
Once the large stone is broken into 5-10 mm fragments, I will then change the settings to 0.2 J and 100 Hz to complete dusting into 100-
500 m dust fragments. I find this approach to be more efficient than going to straight dusting settings because laser pulsations can move
smaller fragments around at a rapid pace, which adds to dusting efficiency.
Large lower pole calyceal stones treated with Ureteroscopy:
Treating a large lower pole stone ureteroscopically has many nuances that makes every stone different. Differences in the lower pole are
affected by the specific ureteroscope used (disposable versus reusable, digital versus fiberoptic) and lower pole anatomic characteristics
(anterior calyceal stone versus posterior calyceal stone, acuteness of the lower pole infundibulum). If the lower pole infundibulum is large
enough to accommodate moving the stone to the renal pelvis or another mid/upper pole calyx is ideal. However, if the stone is too large to
move, I recommend using settings of 0.6 J and 30 Hz, which provides a good balance of fragmentation and dusting.
Once the large stone is fragmented to smaller fragments, the surgeon can choose to move the fragments to a different, more easilyaccessible
calyx, or continue to treat in the lower pole. Once fragments are approx. 10 mm or less, I typically change my laser settings to
0.2 J and 100 Hz. This setting creates a chaotic, yet controlled movement of stones into the laser, which provides very efficient dusting
without much movement of the ureteroscope itself. The laser pulsations create a “suction” effect where fragments are rapidly pulled into
the laser fiber without the need to chase the stones around with the ureteroscope. This is particularly helpful with lower pole stones when
access to all corners of each lower pole calyx can be limited.
The Soltive laser creates a “vacuum-like” behavior that draws stone fragments to the laser fiber, rather than having to move the scope and
fiber to the fragment. This allows for incredibly efficient dusting of stones that are otherwise very difficult to access with the scope and/or
basket.
Treating Large Renal Stones: Soltive’s Versatility for Renal Stones
Dr. Orr’s Strategy
Case Study:
A 35-year-old obese, diabetic male with a history of calcium oxalate and uric acid stones is seen in the office after having a symptomatic, non-obstructing 1.2cm right lower pole renal stone identified via CT scan. KUB in the office does not demonstrate any radiodense stone. Prior attempts at dissolution therapy failed for this patient and is now requesting to be treated surgically. Based on the AUA guidelines, all options are available for treatment of this stone. I prefer to not use ESWL in situations where stones are not visible radiographically because I do not believe stone-free rates are as high as ureteroscopy or PCNL/mini-PCNL in this case scenario.
Assuming the decision is made to treat this patient with ureteroscopy, this case may present a frustrating outcome. Typically, male patients in this age range generally have very tight ureters, if not previously stented. The patient may have ureters that would not accommodate a ureteral access sheath and thus would typically need a stent and later require a staged secondary ureteroscopy.
As is described previously, it may be an option to pass a flexible ureteroscope without an access sheath. If no access sheath is placed, most surgeons would rely on stone dusting with the scope passed next to the wire. However, this may be difficult in tight ureters. Therefore, it is common to place a stent and stage the ureteroscopy.
With the Soltive™ TFL, I commonly backload the flexible ureteroscope to gain access to the most troublesome ureters. This then becomes 100% dependent on dusting because passage in and out the ureter without a safety wire is unsafe and not a feasible way to treat this stone. Using Soltive and a backloaded flexible ureteroscope I can dust this 1.2 cm lower pole stone. I typically use laser settings at 12 watts or less. As a recommendation, the surgeon should pay especially close attention to irrigation flow to ensure there is adequate inflow and outflow during stone ablation.
Stone Fragmentation and Dusting Case Studies
Optimizing Dusting Approach with TFL
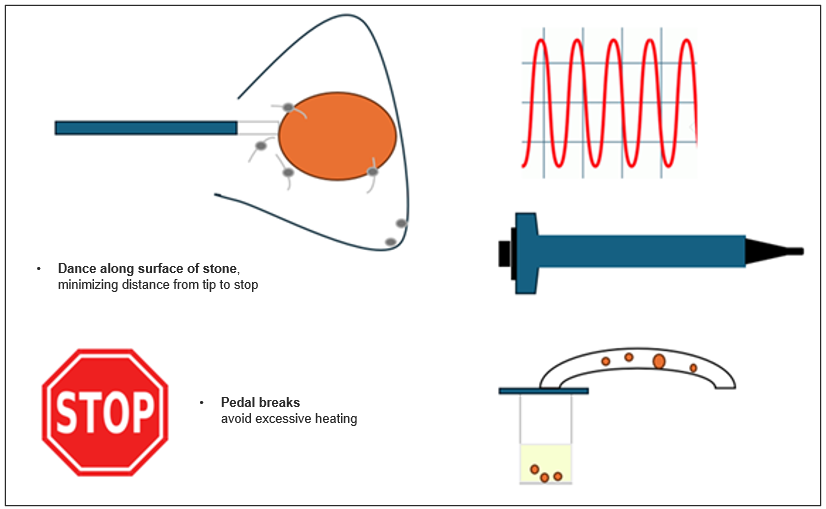
Dr. Chew’s Strategy
High Frequency (10-40 Hz)
• High efficient dusting while limiting heat generation
Ureteral Access Sheath
• Temperature control
• Improved visualization
Suction (Flexible and Navigable Ureteral Access Sheath)
• Improves visualization (compared to no use of suction)
• Improves fragment evacuation (compared to no use of suction)
Stone Fragmentation & Dusting
Dr. Orr’s Strategy
Over the last decade, urologic laser technology has improved in many ways. This provides more options for Urologists to manage urologic stone disease. Laser technology has become more powerful, and the software has improved the delivery of energy to provide more efficient treatment. When considering ureteroscopic treatment of stones, there are two main thought groups of Urologists: those who primarily fragment and basket the stone and those who primarily dust the stone. The Soltive™ laser can be a fragmenting laser and/or a dusting laser per the surgeon’s preference without limitation and can adjust to the needs of majority of cases.
Generally, fragmentation requires higher joules of energy delivered at a lower frequency, whereas dusting generally uses lower joules, with higher frequency. In addition, the overall watts of power need to be considered depending on the location of the stone (bladder vs. ureter vs. kidney) given the understandable concern for heat generation and its possible effects on the surrounding tissue.
General Technique for Ureteral Stones:
Special consideration needs to be taken for ureteral stones due to heat generation and possible impact on the surrounding ureteral tissue. There is less risk of this in the kidney, assuming there is adequate irrigation inflow/outflow keeping overall intrarenal temperature minimally changed from inflow irrigation temperature. In the ureter, however, the heat generation is in close proximity to the tissue without much surrounding fluid for temperature dissipation that heat generation is a particular consideration for ureteral stones. I usually have a maximum laser power of 12 watts in the ureter, but many times have efficient stone management with settings using only 6-8 watts.
The location of the stone within the ureter also affects my settings. For very distal stones, many times I will not open a basket and rely more on dusting as the fragments will passively move into the bladder as the fragments are created. Midureteral stones, I favor more fragmenting with basket extraction as the spontaneous movement of the stone into the bladder is less likely, making basket extraction necessary.
For proximal stones, I either fragment the stones and basket them into the bladder or relocate the stone into the upper pole to dust at higher power settings for more efficient treatment. For ureteral fragmenting, I typically use 1J and 10Hz and often will mix in 0.6 J and 15-20 Hz which also provides some degree of dusting in addition to fragmentation.
General Technique for Renal Stones:
I believe the most efficient way to treat a stone is to treat it within a calyx rather than within the renal pelvis, if possible. If the stone is located within the renal pelvis, it is much more efficient to move the stone into an easilyaccessible calyx (preferably upper pole) if the stone is small enough to fit through the infundibulum. I do this either by simply nudging the stone with the ureteroscope or using a basket, if necessary. If the stone is too large, I generally dust the stone to make it small enough to move, or I fragment the stone into a few pieces to then move each into a selected calyx.
For basketers:
The Soltive TFL can be used similarly to a holmium laser to fragment stones to small enough pieces for efficient basket extraction.
My recommended settings are 1- 1.5 Joules and 10 Hz with short pulse, depending on stone density. This technique is the same for those familiar with fragmentation using Holmium laser technology.
For dusters:
Moving stones to a calyx that provides less space for the stone to move freely offers more efficient dusting capability. The irrigation flow by itself will cause movement of the stone and the laser pulses from the TFL will cause a chaotic, yet controlled, movement of stone to provide incredibly efficient dusting.
I start with settings 0.6 J and 30 Hz on the right pedal to break stones to 5- 10mm fragments, then switch to 0.2 J and 100 Hz to achieve dust fragments between 100 and 500 microns.
Starting with 0.2J/100Hz also works well, but I have found that starting with 0.6 J/30 Hz will more efficiently produce stone fragments small enough so the 0.2 J/100 Hz becomes a more efficient and complete dusting.
Clear Petra Suction Access Sheath
A Strategy for Stone Removal by Dr. Bhojani
Soltive™ Thalium Fiber Laser Dusting with Suction Ureteral Access Sheath
A Strategy for Stone Removal by Dr. Bhojani
Case Details:
• 58 y.o. female
• Past medical hx
– Hypertension
– Diabetes type II
– Known calcium oxalate stone former
• 2 cm left lower pole renal calculi
• Tx: Flexible Ureteroscopy
Case Study: Patient presented with a urinary tract infection and a lower pole left renal stone. A ureteral stent was placed. Three weeks after stent placement, the patient underwent a ureteroscopy to remove the lower pole stone.
Gaining Access was accomplished by using a large suction ureteral access sheath (UAS), which was possible due to the presented ureter
Procedure Steps
• Efficiency of the Suction UAS is obtained by using high pressure irrigation (200- 250mmHg). The high irrigation helps in removal of fragments (they exit the kidney easier; follow the scope out)
• Using a pressure measuring ureteroscope and the Suction UAS (wall suction set at 200) we can maintain low intrarenal pressures (between 10-30mmHg) even with high pressure irrigation
• With high irrigation and low pressures and using a suction UAS, there is constant exchange of fluid within the renal collecting system. This has the potential of keeping the intrarenal temperature low (data pending).
Laser fiber and settings
• A 150-micron laser fiber was used
o This fiber allows for excellent irrigation and ureteroscope deflection
• Laser energy set at 18 watts (0.6J x 30Hz)
• These settings were used as the stone was found to be very hard and difficult to dust.
2cm left lower pole renal calculi
- Placement 12/14Fr ureteral access suction sheath
- Flexible ureteroscope
- Pressure irrigation set at 200-250mmHg
- Wall suction set at 200
- 150 micron SOLTIVE Thulium Fiber Laser fiber
- Laser energy set at 18 watts (0.6 J x 30Hz)
Percutaneous Access Nephrolithotomy (PCNL) Case Studies
PCNL has become a minimally invasive surgery; ambulatory Mini-PCNL for large stones
Jorge Gutiérrez-Aceves, MD; Alejandro Bautista-Pérez-Gavilán, MD
Percutaneous Access Nephrolithotomy (PCNL) is the preferred treatment for large and complex renal stones. Contemporary techniques involve a more refined, minimally invasive approach, using ultrasound (US)-guided access, a miniaturized 16/18 Fr percutaneous tract, and enhanced laser technology with Thulium Laser Fiber (TFL), which allows for more efficient stone dusting and/or fragmentation.
Vacuum-assisted access sheaths also allow for aspiration of stone fragments; help avoid basket and grasper use and minimize intrarenal pressure. Mini-PCNL provides an overall less invasive experience, minimizes intraoperative bleeding, nullifies the need for sutures, and facilitates same-day discharge.
Prior to the procedure, a retrograde ureteral access catheter with extension tubing is placed. The patient is then placed in the prone position and the flank of interest is exposed. The fluoroscopic C-arm is rotated 30° cephalad to provide an adequate view of the calyx of interest.
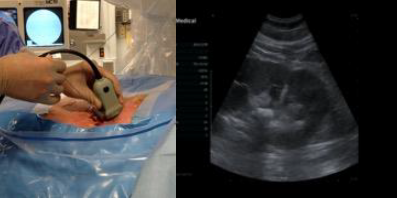
The extension tubing is used to perform an intraoperative retrograde pyelogram. Distension of the calyces provides enhanced visibility during US access. A probe with an attached needle guide is used, and the needle is placed under direct vision into the calyx of interest. US access allows for real-time monitoring of the needle tip.
Confirmation of correct access is aided by fluoroscopic imaging, return of urine and guidewire placement. The guidewire is led all the way down the ureter. The needle is exchanged for a 6-Fr dilator, which is placed all the way past the ureteropelvic junction (UPJ), and the guidewire is exchanged for a super stiff wire. After confirmation the wire is at the level of the bladder, a sub-centimeter skin incision is made. Serial dilators are then used, dilating the fascia up to 14 Fr. A 16 or 18 Fr access sheath with suction capability is used.
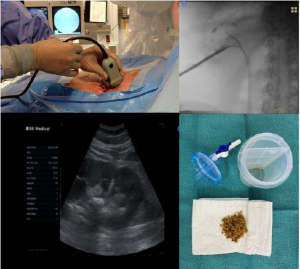
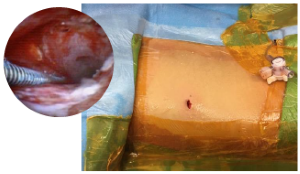
PCNL has become a minimally invasive surgery; ambulatory Mini-PCNL for large stones (continued)
Jorge Gutiérrez-Aceves, MD; Alejandro Bautista-Pérez-Gavilán, MD
After placement over the wire, the sheath obturator is removed, leaving the safety wire in place. The sheath endcap and suction are placed. The 12-Fr mini nephroscope is inserted to obtain endoscopic access and identify the stone in the renal calyx.
Once endoscopic access has been obtained, TFL lithotripsy is carried out. The energy settings should be adjusted according to the stone’s composition; however, I recommend 20-30 watts in the renal pelvis and intrarenal system; my preferred setting for large stones is 1.5 J with 20 Hz in fragmentation mode in short pulse. For Mini-PCNL I use 365 mc fiber, this allows me to use the same fiber through a flexible ureteroscope in an antegrade manner if needed
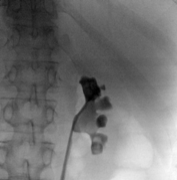
Manipulation of the sheath to place the tip near the stone fragments allows for optimal suction. Stone dust and stone fragments are actively suctioned away from the field of view. Then, all calyces and the UPJ are inspected to see that all fragments have been suctioned. Finally, an antegrade nephrostogram is performed prior to stent placement. The stent is placed under fluoroscopic guidance ensuring the distal curl is in the bladder, and the proximal curl is developed endoscopically and confirmed on fluoroscopy.
A final nephrostogram is done to ensure adequate drainage of the kidney. As the access sheath is removed, a final inspection of the tract is done endoscopically to ensure there is no injury or significant bleeding. The sub- centimeter incision does not require closure; a simple gauze sterile dressing is placed.
Case Study:
This 58-year-old patient presented with a 5-centimeter, left partial staghorn calculus. Mini-PCNL access was obtained, and the tract was dilated to 18 Fr. Using the vacuum-assisted access sheath, TFL lithotripsy was performed with adequate fragmentation and dusting using standard settings1.5 J 20 Hz is my preferred setting for Mini-PCNL, I use short pulse, especially in large stones. Keeping a continues irrigation and outflow, I have never seen a thermal lesion to the kidney. With the debris being suctioned through the access sheath.
After proper inspection of the ureter, UPJ, and maneuvering the sheath to visualize the renal calyces to confirm that all fragments were suctioned, an antegrade stent was placed, with adequate distal coiling in the bladder and proximal coiling in the kidney. A nephrostogram was performed to ensure drainage was adequate. The sheath was removed, with no significant bleeding or injury noted. The incision was covered with sterile gauze dressing. Total surgical time was 105 minutes, and the patient was discharged on the same day.
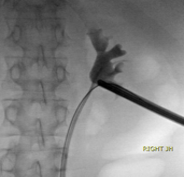
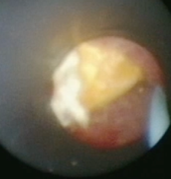

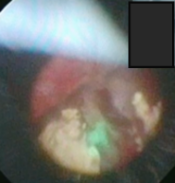
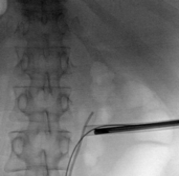
Laser Enucleation Case Studies
Laser Enucleation of the Prostate using Soltive™ Thalium Fiber Laser
Dr. Bhojani’s Strategy
For Large Prostates:
Large prostate glands (>100grams) present a significant challenge
• Increased chance of bleeding
• Longer duration of surgery
Preferred option if available is anatomical endoscopic enucleation of the prostate
• Durable
• Pathological specimen
• Excellent hemostasis when performed with laser energy
• Complications are rare
• This should ideally be performed with a laser
26 Fr continuous flow resectoscope and 550 micron Soltive Thulium laser fiber
Soltive Thulium Fiber Laser Settings:
• 1J x 60 Hz for enucleation (short pulse)
• 1J x 30 Hz for hemostasis (long pulse)
Case Details:
• 78 year old male
• Past medical History: None
• Medication: Tamsulosin
• Retention x 6 months: Failed trail of void
• Pre-op plan:
-Prostate volume measurement, 130 grams
-Cystoscopy
Prostate Enucleation using the SOLTIVE
Key points to Soltive Thulium Fiber Enucleation of the Prostate:
- Stay away from the tissue. About 0.5 cm away is best (no touch technique).
This will avoid tissue charring. - Find the plane and stay on it.
- Try to recognize different types of tissues (adenoma vs prostatic capsule).
- Stop bleeder as you go. Don’t wait until the end.
Soltive Laser Enucleation of the Prostate
Dr. Bhojani’s Strategy
Disclaimer:
The Content of this presentation the views and opinions of Dr. Ben Chew, Dr. Mitchell Humphreys, Dr. Brian Orr, Dr. Michael Lipkin, Dr. Naeem Bhojani, and Dr. Jorge Gutierrez-Aceves and does not necessarily represent the views or opinions of Olympus. This Content does not constitute medical or legal advice and should not be considered as a substitute for carefully reading all applicable labeling, including the Instructions for Use. Please thoroughly review the relevant user manual(s) for instructions, warnings, and cautions.
Information regarding competitor products is presented to the best of our knowledge as of the date of presentation. This material does not constitute medical or legal advice and should not be relied upon as such. It should not be considered as a substitute for carefully reading all applicable labeling, including the Instructions for Use. Please thoroughly review the relevant user manual(s) for instructions, warnings and cautions. Techniques, instruments and setting can vary from facility to facility, and it is the clinician’s decision and responsibility in each clinical situation to decide which mode and settings to use.
All trademarks, logos and brand names are the property of their respective owners
Dr. Ben Chew, Dr. Mitchell Humphreys, Dr. Brian Orr, Dr. Michael Lipkin, Dr. Naeem Bhojani, and Dr. Jorge Gutierrez-Aceves, the authoring physicians of this presentation, are paid consultants to Olympus Corporation of the Americas.
- Keyword
- Content Type




