Gastrointestinal scope series
Clinical Benefits of Endocyto in Endoscopic Diagnosis of Esophageal Lesions and Tips for its Use
Dr. Kenichi Goda
Showa University Koto Toyosu Hospital, Digestive Diseases Center
I. Clinical advantages of Endocyto
Endoscopic diagnosis with high confidence made possible by in vivo cell observation
The only conventional method for establishing a definitive diagnosis of gastrointestinal lesions has been histopathological microscopic examination of specimens obtained by biopsy. Endocyto, an ultra-high magnification gastrointestinal endocytoscope with a maximum magnification of 520× (when using OEV262H), has made it possible to capture histological structure directly by in vivo observation at a cellular level after double staining of nuclei and cells1). This should allow endoscopists to make a diagnosis with a higher confidence than is possible with a conventional magnifying endoscope at the maximum magnification of about 80× to 100×.
In the diagnosis of esophageal lesions, endocytoscopy (EC) using Endocyto is expected to be particularly useful for differentiating between neoplasia and non-neoplasia. For example, the existence of ultra-minute squamous neoplasms sized about 1–3 mm has been attracting attention in recent years2–4). Since they occur at a very early stage, morphological abnormalities of the intraepithelial papillary capillary loop (IPCL) that can be captured by magnifying narrow-band imaging (NBI) observation may not have severe abnormality, even when they are histologically cancers. Even after attempting biopsy, it may be difficult to prepare appropriate tissue specimens because the lesions are very small. By contrast, EC observation may lead to accurate diagnosis by capturing atypical nuclei and cells in the whole minute lesions.
Vascular findings obtained by endocytoscopy with NBI observation may be useful for invasion depth diagnosis
When estimating the invasion depth of esophageal cancer, observation of nuclei and cells using Endocyto is not expected to provide any advantage over conventional magnifying NBI observation in terms of improving the ability of invasion depth diagnosis based on superficial vessels. However, EC with NBI provides vascular images magnified up to about 5 times the size of conventional magnifying NBI (Figure 3). This allows individual vessels to be clearly visualized. It has been reported that the vascular caliber in superficial carcinoma correlates with its depth of invasion5), and the B3 vessel (a finding suggestive of SM2 or deeper invasion) is defined as having a caliber larger than 60 μm according to the classification of the Japan Esophageal Society6). However, these vascular calibers are measured on histopathological samples and not in vivo. Therefore, if the vascular calibers can be precisely measured by EC with NBI, it may be possible to investigate the relationship between the depth of invasion and the vascular caliber.
II. Procedure and hints for observation using Endocyto
Obtaining a well-stained image of nuclei and cells
To obtain useful diagnostic information by EC in the esophagus, it is important to acquire well-stained images of nuclei and cells in the target site.
As the staining procedure (Table), first perform non-magnifying observation to trace abnormal findings, such as superficial irregularities and redness under white light or brownish area under NBI, then perform magnifying NBI observation to make a characterization based on the findings of IPCLs. Next, to confirm the target site to be stained for EC observation, undo the magnification to the non-magnifying level and apply the staining solution covering the lesion and its surrounding tissue.
|
[1] After characterization by magnifying NBI observation, return to the non-magnifying white light observation. Procedure for stainining nuclei and cells in endocytoscopy of the esophagus |
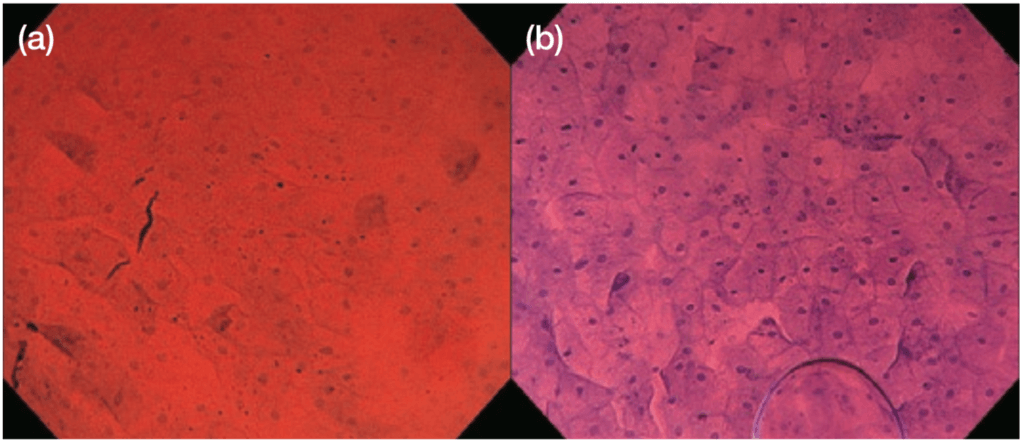
We prepare 11 cc of a mixture of 0.05% crystal violet 10 cc and 1% methylene blue 1 cc as the staining solution7). Since the risk of induction of genetic abnormality by in vivo staining with 1% methylene blue has previously been reported8), we use methylene blue at extremely low concentrations (0.1% or less) to ensure safety. Before applying the staining solution, wash the mucus adhering to the mucosal surface by rinsing with 50–60 cc of water. To prevent over-dilution of the staining solution that would lead to insufficient staining, ensure that after rinsing any residual water is completely removed by aspiration. After applying the prepared staining solution at a single dose of 1–2 cc, start EC observation, gradually increasing the magnification and focusing on the target site. If staining is insufficient even at 1–2 minutes after applying the solution, apply additional staining solution (Figure 4).
While sufficiently good staining is obtained by one application in the esophagus, lesions in the stomach and duodenum tend to be more difficult to stain. In principle, we do not remove the staining solution remaining in the esophagus by aspiration. This is because the residual staining solution can be expected to enhance the stainability of cells, and the image quality may deteriorate if the superficial mucosal layer cells detach and adhere to the lens because of aspiration. Stained images are considered to be most suitable for observation at 2–3 minutes after the beginning of EC observation. Thereafter, concentrate on obtaining EC images of good quality within about 5 minutes because cells progressively detach from the superficial mucosal layer over the course of time.
During EC observation using Endocyto, the color tone is calibrated at red −8 and blue +8 by the video processor that is part of the system unit. This enhances the quality of images of stained nuclei and cells, and allows Endocyto images suitable for diagnosis to be obtained even when using a staining solution containing methylene blue at low concentration.
Scope manipulation of the Endocyto (approach and magnification adjustment)
With Endocyto, the magnification can be adjusted serially from non-magnifying to the maximum magnification of 520× by manipulating the zoom lever. Although this zoom system requires more delicate lever manipulation than a conventional magnifying endoscope (maximum magnification: about 100×), the delicate manipulation will make it possible to focus on the target area by gradually increasing the magnification from non-magnifying observation to the conventional magnifying NBI observation. More specifically, the procedure (Figure 5) involves gradually bringing the scope closer to the site including the abnormal vessel detected by magnifying NBI observation before staining, while at the same time increasing the magnification from about 100× (cells cannot be seen) to 200–300× (a population comprising several hundred cells can be seen). Approach more closely while focusing on the site where the structure/cell within that cell population has a higher grade of atypia to finally perform contact endoscopy, and acquire EC images when the maximum magnification is reached.
Thus this procedure, which consists of slowly advancing the scope while adjusting the magnification until the endoscope comes into contact with the mucosal surface at the maximum magnification, has several advantages, not just because it would be difficult to miss the target but also because bleeding risk in the mucosa is reduced. If bleeding occurs during EC observation, the observation field is markedly disturbed because individual red cells are visualized. If you miss the target while increasing the magnification, it is necessary to decrease the magnification and try again to approach from a more distant view. In this case, avoid rapid magnification adjustment and decrease the magnification while gradually separating the scope from the mucosal surface. When the target is captured again within the visual field, repeat the approach from near view while increasing the magnification to refine the observation site. However, in the esophagus, which is a narrow and linear lumen organ, the approach has to be made from a tangential direction to the lesion. For this reason, the elasticity of the mucosal surface is enhanced by reducing distensibility of the esophageal wall with inspiration during close-up/contact observation. By doing this it becomes possible to perform contact endoscopy because the mucosal surface is deformed by slightly pushing the tip of the scope onto it. However, it is usually difficult to acquire a good still image while stabilizing the focal length when the scope is positioned away from the mucosa during observation at a magnification of 200–300×. At this level of magnification, I consider it important to spot the sites showing a high grade of structural atypia and to focus on these during observation.
Capturing the characteristics of the lesion in comparison with normal mucosa
Similar to conventional endoscopic diagnosis, the processes of EC diagnosis are based on comparison with normal findings.
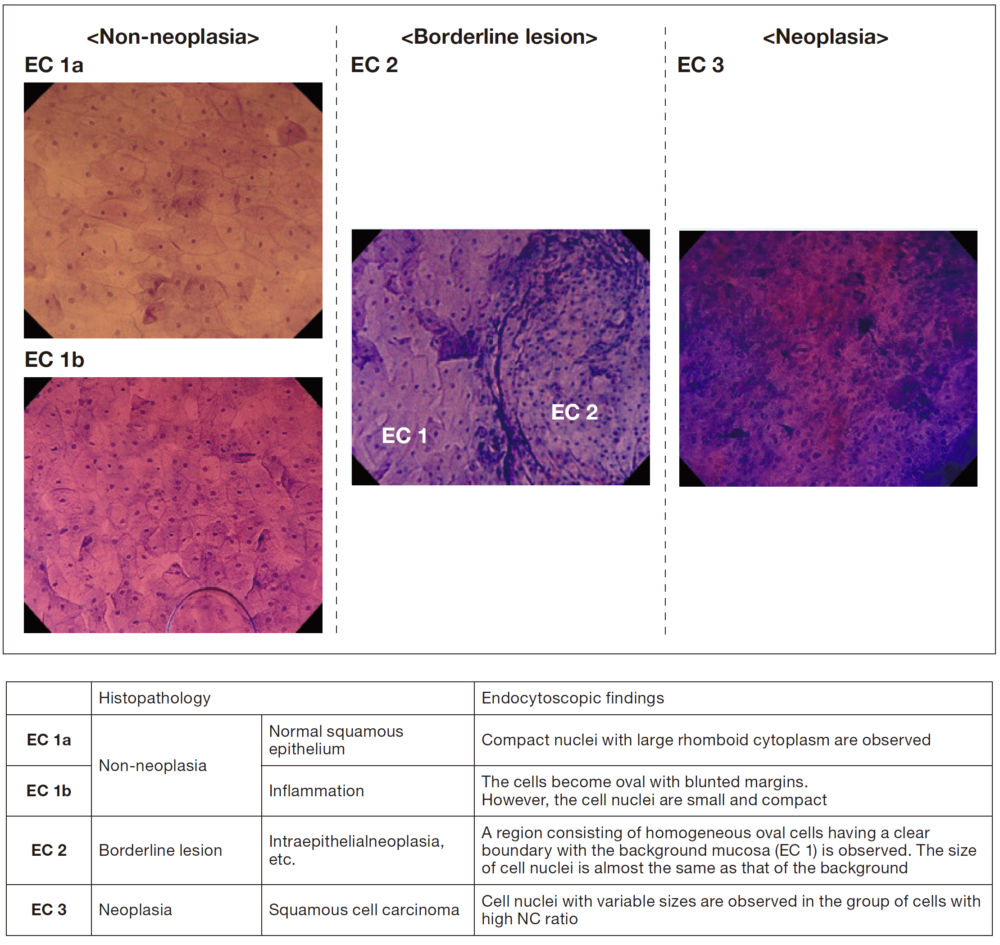
To make an endoscopic diagnosis of esophageal lesions based on EC images, the EC classification (Figure 2) will be useful. Considering that the esophagus has no glandular structure, I consider it easy to learn this simple diagnosis system, which facilitates diagnosis by conducting an evaluation in the order of the density, size, and shape of the cytoplasms nucleus. However, it is important to become familiar with EC images obtained at much higher magnifications compared with conventional magnifying endoscopy. As a tip for this, after sufficiently staining the lesion including the surrounding normal mucosa, first closely observe the density, size, and shape of the nuclei in the normal mucosa at the boundary of the lesion and memorize their images. Thereafter, evaluation and diagnosis based on the EC classification can be performed by identifying the presence of clear boundaries and the characteristics of EC images within the lesion (especially the shape of cytoplasms, cell density, and the size/shape irregularity of nuclei) in comparison with EC images of the normal mucosa, while gradually entering into the interior of the lesion.
For EC observation at the maximum magnification when conducting contact endoscopy, since there is no space between the magnifying lens and the mucosal surface that would allow the illumination light to enter, the image appears to be dark owing to a lack of light. For resolving this problem, we attach a silicon rubber tip at an angle to the endoscope tip (firmly fixing it with vinyl tape) and create a gap on the side of the illumination lens to allow the illumination light to be directed onto the magnifying lens surface, particularly during contact endoscopy.
III. Expectations regarding other clinical applications of Endocyto
Detection of NBI-negative early-stage cancers and diagnosis of non-erosive gastroesophageal reflux disease
I believe that additional clinical applications of EC observation for endoscopic diagnosis of the upper gastrointestinal lesions may come to light in future assessments. For example, for follow-up after endoscopic treatment for esophageal cancer, the results of our study suggested that the risk of missing precancerous/early- stage cancer lesions in the esophagus showing multiple Lugol- voiding lesions may be higher in magnifying NBI observation than in Lugol’s iodine staining9). Even in precancerous/early- stage cancer lesions that cannot be detected by vascular pattern-based diagnosis using magnifying NBI observation alone, some cellular atypia is detected by additionally performing EC observation, potentially leading to optimization of the examination interval and reducing the risk of missing such lesions.
In gastroesophageal reflux disease, symptoms of heartburn and acid reflux may appear even when conventional endoscopy reveals no abnormal findings. It may become possible to make an endoscopic diagnosis by performing EC observation in such patients to detect abnormalities at the histological level, such as infiltration of inflammatory cells into the squamous epithelium layer.
On the other hand, for EC observation of the stomach, similarly to the esophagus, a double staining method using a mixture of crystal violet and methylene blue has been attempted, but the difficulty in obtaining sufficient staining remains a problem. However, I expect that in the future it will be possible to differentiate between neoplasia and non-neoplasia by endoscopic diagnosis using Endocyto, similar to diagnostic biopsy, even in the stomach, when a computer-aided diagnostic system currently being developed for the colorectum10) is applied and made practicable for use in the stomach.
[References]
1) Inoue H, et al. In vivo observation of living cancer cells in the esophagus, stomach, and colon using catheter-type contact endoscope, “Endo-Cytoscopy system”.Gastrointest Endosc Clin N Am 2004; 14: 589-594.
2) Inoue H, et al. Ultra-high magnification endoscopy of the normal esophageal mucosa. Dig Endosc 1996; 8: 134-138.
3) Inoue H, et al. Ultra-high magnification endoscopic observation of carcinoma in situ of the esophagus. Dig Endosc 1997; 9: 16-18.
4) Goda K, Dobashi A, Yoshimura N, et al. Clinicopathological features of narrow-band imaging endoscopy and immunohistochemistry in ultraminute esophageal squamous neoplasms. Dis Esophagus 2014; 27: 267-275.
5) Santi EG, Inoue H, Ikeda H, et al. Microvascular caliber changes in intramucosal and submucosally invasive esophageal cancer. Endoscopy 2013; 45: 585-588.
6) Oyama T, Inoue H, Arima M, et al. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: magnifying endoscopic classification of the Japan Esophageal Society. Esophagus 2017; 14: 105-112.
7) Inoue H, Yokoyama A, Kudo SE. Ultra-high magnifying endoscopy: Development of CM double staining in endocytes and its safety. Nihon Rinshou 2010; 68: 1247-1252.
8) Oliver JR, Wild CP, Sahay P, et al. Chromoendoscopy with methylene blue and associated DNA damage in Barrett’s oesophagus. Lancet 2003; 362: 373-374.
9) Goda K, Dobashi A, Yoshimura N, et al. Narrow-band imaging magnifying endoscopy versus lugol chromoendoscopy with pink-color sign assessment in the diagnosis of superficial esophageal squamous neoplasms: a randomised noninferiority trial. Gastroenterol Res Pract 2015; 2015: 639462.
10) Misawa M, Kudo SE, Mori Y, et al. Characterization of colorectal lesions using a computer aided diagnostic system for narrow-band imaging endocytoscopy. Gastroenterology 2016; 150: 1531-1532.