Colonoscope Series
History of Development of the Colorectal Endocyto, and its Anticipated Ability in the Diagnosis of Colorectal Lesions
Dr. Shin-ei Kudo
Showa University Northern Yokohama Hospital, Digestive Disease Center
Endocyto observation can provide information comparable to that obtained by biopsy
Before endoscopes began to be used to examine the digestive tract, around 150 years ago, the gold standard for diagnosing gastrointestinal cancer was histopathological diagnosis by microscopic evaluation of structural and cellular atypia in tissue samples stained with hematoxylin and eosin (HE). Endoscopists have long anticipated access to detailed diagnostic information by examining structural and cellular atypia via endoscopy in vivo. In the 1990s, in collaboration with Olympus we developed magnifying endoscopes enabling endoscopists to make in vivo pit pattern diagnoses. These endoscopes have now been marketed. Pit pattern diagnoses are based on assessment of the morphology of ductal openings on colorectal tumor surfaces using stereo microscopes. This development led to the widespread use of magnifying endoscopes capable of up to 80–100× magnification, thus enabling evaluation of structural atypia of the mucosal surface layer. However, even more definitive diagnosis of cancer requires identification of cellular atypia and examination of cell nuclei. Consequently, in conjunction with Olympus, we embarked upon development of an ultra-magnifying endoscope (Endocyto) that would enable in vivo assessment of cells and their nuclei in colorectal mucosa. The Endocyto colonoscope, which allows ultra-magnifying endoscopic observation (endocytoscopy: EC) at up to 520× magnification (when using with 26 inch monitor: OEV262H), is now available to clinicians. A single scope enables both regular non-magnifying and magnifying observation. Additionally, further EC observation at higher magnifications can be achieved through a series of zoom lever manipulations, making it possible to assess cellular atypia in addition to the structural atypia that can be assessed using a conventional endoscope. That the novel diagnostic modality of EC can provide information comparable to that obtained by histopathological examination of biopsy samples, in vivo and on-site, is expected to make a major clinical contribution to investigation of patients with colorectal lesions.
EC improves ability of endoscopic diagnosis and helps to clarify the mechanisms by which cancer metastasizes
When using conventional magnifying endoscopes to diagnose and assess colorectal tumors, differentiating between benign and malignant tumors and ascertaining depth of cancer invasion have been achieved by predicting the tissue type in the deeper layers and estimating the depth of infiltration solely on the basis of information about structural atypia in the mucosal surface layer. In contrast, the EC observation enabled by Endocyto together with the technique of staining cells and their nuclei allows assessment of nuclear morphology, extent of swelling, and degree of cellular atypia. We have proposed an EC classification (Figure 1) in which the characteristics identified by EC observation are divided roughly into three classes or more finely into five classes and correlated with predicted tissue types1, 2). We have also demonstrated that EC enables more accurate diagnosis than conventional endoscopic diagnosis and that EC observation is non-inferior to diagnosis by biopsy regarding ability of differentiating between neoplasia and non-neoplasia3).
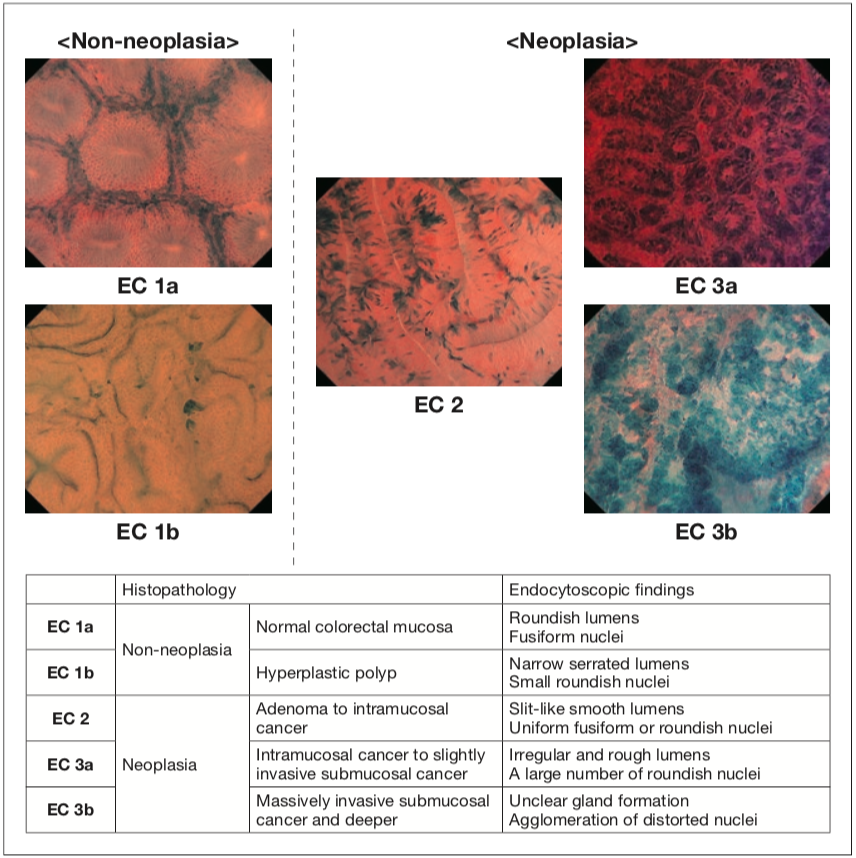
Figure 1 EC classification for the colorectum [neoplasia EC 3a: reproduced from reference 2; Table: reproduced with modification from reference 1 with permission from © Georg Thieme Verlag KG.]
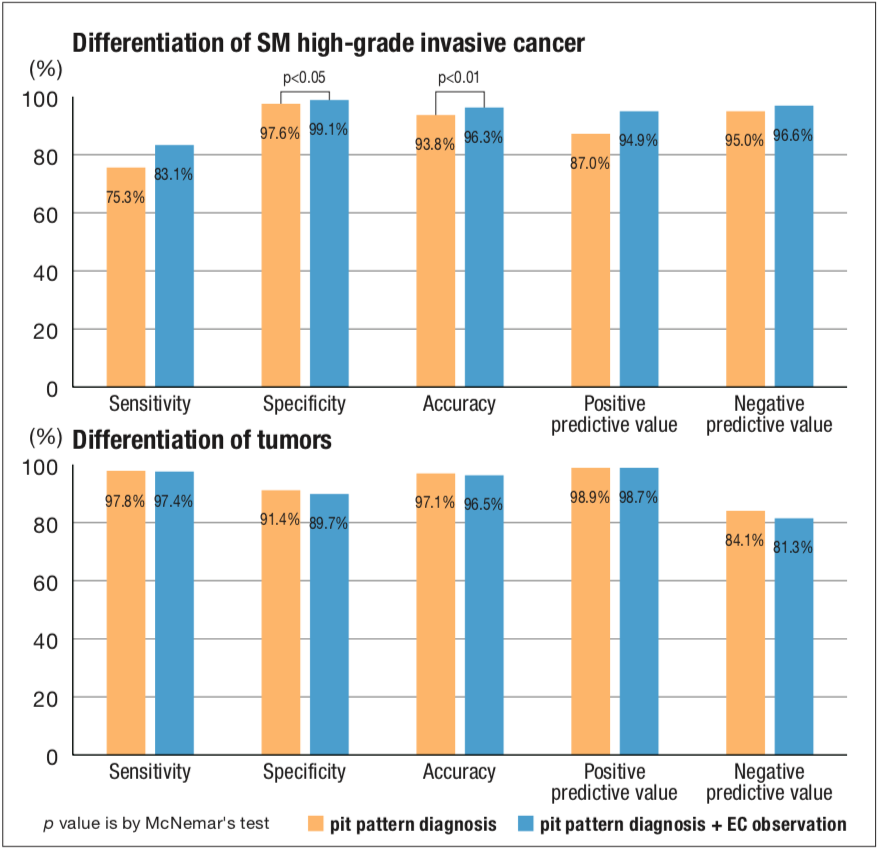
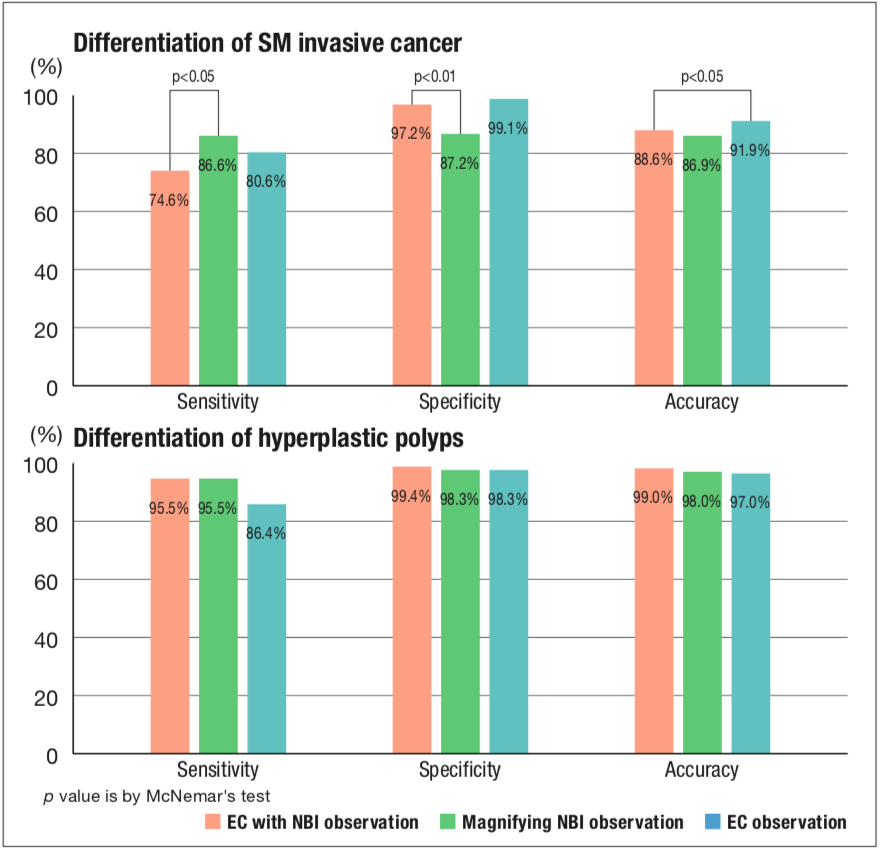
It has been shown that observation of magnifying chromoendoscopy provides an additional level of ability regarding assessment of depth of invasion over that achieved by pit pattern diagnosis (Figure 2)4). It is difficult to distinguish moderately-differentiated adenocarcinoma, with its higher risk of metastasis, from well-differentiated adenocarcinoma by observation using conventional magnifying endoscopes, whereas highly accurate diagnosis may be possible by EC observation; verification of this is now underway. It is anticipated that a biopsy, currently needed for a definitive diagnosis of cancer, will become unnecessary and treatment plans, including the options of endoscopic treatment or surgical intervention, will be determined on the basis of endoscopic diagnosis using Endocyto in real time.
Endocyto is capable of performing EC with narrow band imaging (NBI), meaning that microvascular structures such as vasodilation, vasoconstriction and changes in continuity can be evaluated. We have proposed an EC-V classification whereby these findings are divided into three classes and are working to verify its usability as a supplemental diagnostic index to the EC classification (Figure 3)5). By employing EC with NBI, erythrocyte behavior within blood vessels and speed of blood flow can also be captured in vivo, these being characteristics that cannot be gauged from histopathological findings in resected specimens. It is expected that this form of direct observation will also help to elucidate the mechanisms of metastasis and tumor angiogenesis in greater detail.
Early detection and treatment of colorectal cancer are anticipated
It is expected that Endocyto will have many important advantages for clinical practice around colorectal cancer. More accurate endoscopic diagnoses, including evaluation of the degree of malignancy, will be made. This will enable earlier detection of high-risk colorectal cancer and development of more appropriate treatment and follow-up plans that incorporate the principles of preventive medicine. Additionally, given that it will be possible to make a definitive diagnosis without having to examine biopsy specimens pathologically, the process of medical care will be accelerated and the burden on patients subsequently lightened. In particular, avoiding unnecessary biopsies will be of great advantage to patients at high risk of bleeding.
An automated diagnosis system6, 7) equipped with artificial intelligence and based on nuclear morphology and microvascular morphology extracted and processed from EC images is currently under development. If this system is introduced into clinical settings in the future, it will be easy to rapidly and extremely accurately diagnose colorectal cancer. Given that reducing the mortality rate of colorectal cancer is an urgent issue, the clinical merit of such improvements will be very welcome.
[References]
1) Kudo SE, Wakamura K, Ikehara N, et al. Diagnosis of colorectal lesions with a novel endocytoscopic classification – a pilot study. Endoscopy 2011; 43: 869-875.
2) Misawa, M., & Kudo, S. Endocytoscopy: Small advanced cancer (de novo). In H. Tajiri (Ed.), NBI/BLI/LCI Endoscopic Atlas Based on New Criteria and Classifications. 262-265. Nihon Medical Center, 2016.
3) Mori Y, Kudo S, Ikehara N, et al. Comprehensive diagnostic ability of endocytoscopy compared with biopsy for colorectal neoplasms: a prospective randomized noninferiority trial. Endoscopy 2013; 45: 98-105.
4) Kudo SE, Mori Y, Wakamura K, et al. Endocytoscopy can provide additional diagnostic ability to magnifying chromoendoscopy for colorectal neoplasms. J Gastroenterol Hepatol 2014; 29: 83-90.
5) Kudo SE, Misawa M, Wada Y, et al. Endocytoscopic microvasculature evaluation is a reliable new diagnostic method for colorectal lesions (with video). Gastrointest Endosc 2015; 82: 912-923.
6) Misawa M, Kudo SE, Mori Y, et al. Characterization of colorectal lesions using a computer-aided diagnostic system for narrow-band imaging endocytoscopy. Gastroenterology 2016; 150: 1531-1532.
7) Mori Y, Kudo SE, Wakamura K, et al. Novel computer-aided diagnostic system for colorectal lesions by using endocytoscopy (with videos). Gastrointest Endosc 2015; 81: 621-629.