Robert Staroń, MD, PhD,
Consultant Gastroenterologist
Department of Gastroenterology
and Hepatology with Internal
Disease Unit Clinical Voivodship
Hospital No. 1Rzeszów, Poland

Łukasz Krupa, MD, PhD,
Consultant Gastroenterologist MRCP UK
Department of Gastroenterology and Hepatology with Internal Disease Unit Clinical Voivodship Hospital No. 1Rzeszów, Poland
Introduction
In our center, we have been performing interventional EUS procedures endosonography since 2015. We perform approximately 55 EUS-guided biliary drainage (EUS-BD) procedures annually.
Challenges in Primary Malignancies of Pancreato-biliary Tract
Primary malignancies of pancreato-biliary tract result in obstructive jaundice. These malignancies are often detected in advanced stages and are only suitable for palliative treatment. Endoscopic retrograde cholangiopancreatography (ERCP) is the procedure of choice in such patients. However, even in expert hands ERCP might fail in 3%-5% of cases. In case of unsuccessful ERCP, percutaneous transhepatic biliary drainage (PTBD) is performed. However, this procedure is associated with significant morbidity (in up to 30% of cases), with patients experiencing discomfort due the presence of the percutaneous drain and the need for re-interventions.
EUS-guided Biliary Drainage (EUS-BD)
In the past two decades, EUS-guided biliary drainage (EUS-BD) has emerged as an alternative to PTBD. In brief, EUS-BD can be divided into three techniques: the rendezvous technique (EUS-REN) and transluminal stenting technique (EUS-TL), which usually uses a transgastric (hepaticogastrostomy, HGS) or transduodenal (choledochoduodenostomy, CDS) approach.
HANAROSTENT® BPD for Hepaticogastrostomy Procedure
Here we aim to present our experience with the hepaticogastrostomy procedure. HGS is quite a complex endoscopic procedure that requires proficiency in both ERCP and EUS endoscopic techniques. It also requires experienced nursing staff and advanced accessory devices.
The procedure requires a special partially covered self-expanding metal stent. For our hepaticogastrostomy procedures, we use Hanarostent® BPD (MI Tech). We have so far placed 45 of these stents, and find them to be a good choice as a treatment option.
Procedure
The procedure requires few more endoscopic accessories such as an 19G EUS needle, ERCP wire and cystotome. We don’t routinely perform balloon dilatation for the procedure. We also don’t routinely place a plastic pigtail stent into the lumen of deployed hepaticogastrostomy stents.
The procedure takes approximately 25 minutes. We perform it under endoscopic, fluoroscopy and EUS guidance. Hepaticogastrostomy can be performed in a patient with dilatation of the intrahepatic ducts measuring minimum 8 mm. The dilated left intrahepatic duct is located under endosonographic guidance, using therapeutic linear echoendoscope. As a next step, the duct is punctured with a standard 19 G EUS needle, the bile is then aspirated for confirmation and a cholangiogram is performed.
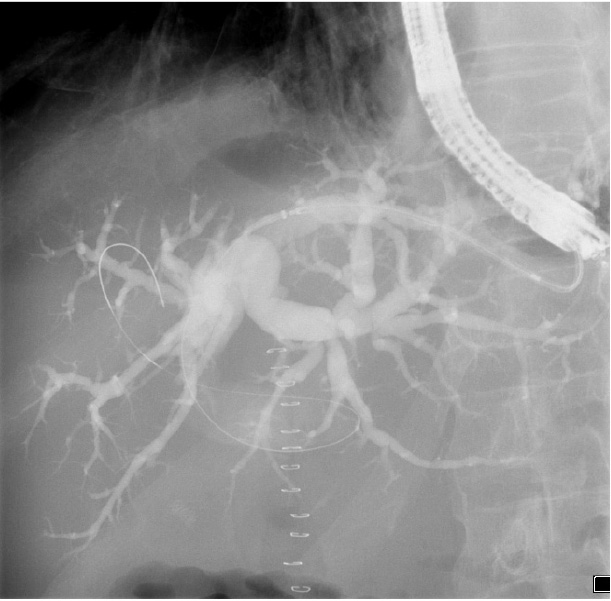
In a further step, we insert a 0.035 guidewire into the dilated bile duct through the needle under EUS and fluoroscopic guidance. The needle is removed while the wire is kept stable in the bile duct. Subsequently, a fistula between the stomach and left intrahepatic duct is created using 6Fr cystotome under EUS/ floroscopic guidance. The cystotome is then removed and a stent is inserted through the therapeutic channel of the scope over the wire into the bile duct (Picture 1).
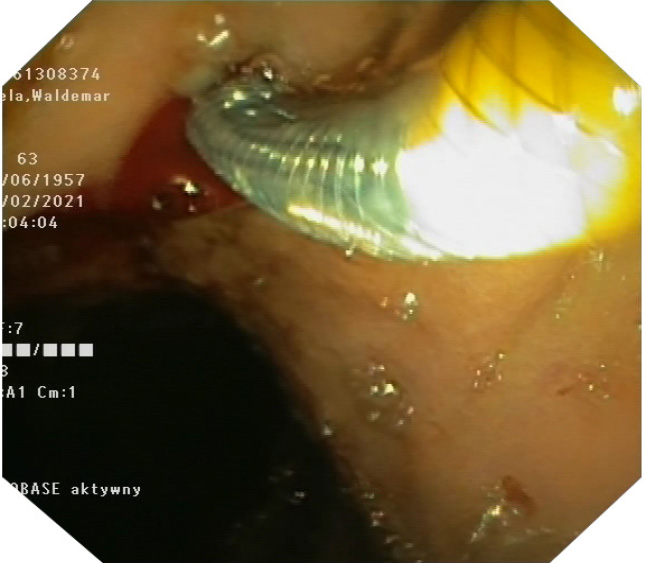
During the stent deployment, we find it very important to have access to fluoroscopic, endosonographic and endoscopic guidance simultaneously. The first part of the stent is opened inside the bile duct under endosonographic and fluoroscopic guidance. In our experience, any attempt to obtain endoscopic views during this step may lead to loss of position. Once we make sure that the first part of the stent is opened in the bile duct under fluoroscopy, we stop deploying it and move to endoscopic view. The yellow marker is visualized and the remaining part of the stent is opened under endoscopic view (Picture 2). The delivery device and the wire are removed carefully. The stent starts opening gradually. We do not perform stent dilatation routinely, it usually opens up entirely in 24 hours, with sufficient biliary drainage.
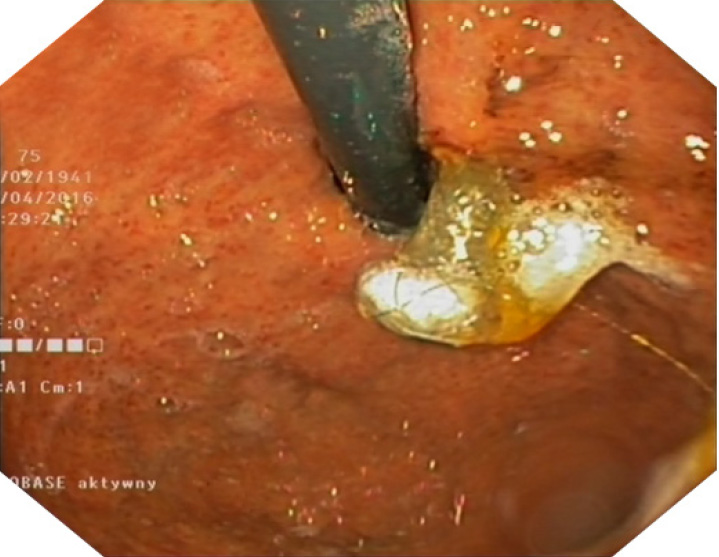
The stents are available in two lengths: 8 and 10 cm. This is a partially covered stent comprising two 2 parts: covered and uncovered. The uncovered part is placed in the intrahepatic duct, which prevents migration and does not block branches of the bile duct. The covered part prevents bile leakage from the hepatic duct into the peritoneal cavity. There is a flange head at the end of the covered part of the stent that prevents migration of the stent out of the stomach (Picture 3).
Evaluation of HANAROSTENT® BPD
We do not report significant problems with the stability of the stent. So far, we have only one case of delayed, spontaneous migration. The delivery device is easy and intuitive to use. The advantage of the stent is the option of placing the stent back into the sheath when necessary, which allows position change and re-deployment of the stent once it is stable.
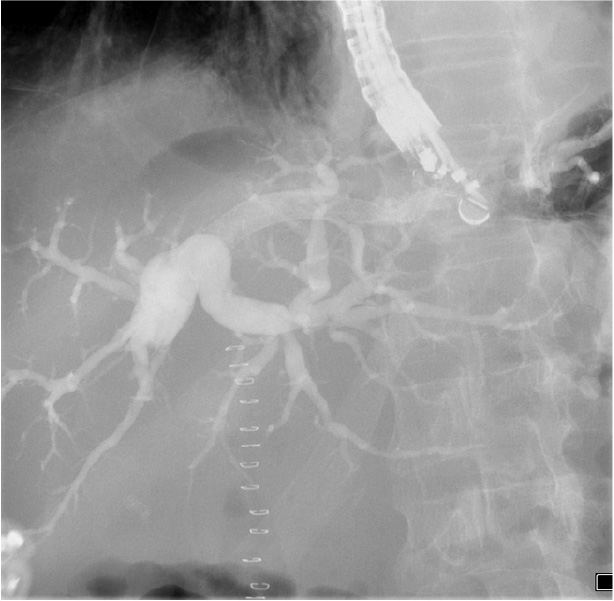
The stent is seen clearly under endosonography and fluoroscopic guidance. It has three radiopaque markers at every end, and in the middle. The middle marker separates the covered part of the stent from the uncovered part. This allows precise assessment of the stent position and deployment status under fluoroscopy (Picture 4). The yellow marker on the applicator is seen clearly endoscopically (Picture 2).
There are possible complications associated with every procedure. During our experience of using the stents, we had one case of bleeding and one case of deployment of the stent in the peritoneal cavity. In the first case, bleeding occurred during the procedure and was stopped endoscopically. In the second instance, we had to reposition the stent laparoscopically. The hepaticogastrostomy is a palliative procedure, but the stent may become occluded with time. So far, all the patients who have returned with stent occlusion were successfully managed endoscopically by insertion of another stent into the stent (metal or plastic).
Conclusion
In summary, Hanarostent® BPD (MI Tech) is an important endoscopic accessory for endoscopic management of obstructive jaundice after unsuccessful ERCP. The device is intuitive and reliable. It is clearly seen in three modalities throughout the entire deployment process: endoscopic, fluoroscopic and endosonographic.
As medical knowledge is constantly growing, technical modifications or changes of the product design, product specifications, accessories and service offerings may be required.
- Keyword
- Content Type