Introduction
Surgical treatment of morbid obesity, known as bariatric surgery, is currently the only effective treatment for the most severe forms of this disease. However, it should not be forgotten that bariatric surgery, like any surgery of the gastrointestinal tract, carries a significant risk of complications, including severe ones, sometimes life-threatening. Therefore, surgical treatment is limited to the most severe forms of obesity, defined as morbid obesity, characterized by a body mass index (BMI) of 40 kg/m2 or 35 kg/m2 with significant metabolical comorbidities.
The abundance of different bariatric procedures quite clearly demonstrates that we have not developed an optimal and universal surgical solution. However, almost all procedures performed nowadays have one common denominator – a manual or mechanical suture line within the gastrointestinal tract as the result of the resection of a given organ or to create an anastomosis. Regardless of the quality of technology used, experience and skills of the operator performing the procedure, it always carries a risk of complications related to healing of the anastomosis. One of the most lifethreatening complications is leakage of gastric or intestinal contents through the disrupted suture line.
In recent years, sleeve gastrectomy has become one of the most popular bariatric procedures. It is perceived as relatively simple technically, providing good results in terms of weight loss. During this operation, a narrow tube is created along the small curvature of the stomach. Resection line follows its long axis and a significant portion of the stomach body and fundus is removed with a stapler. A major disadvantage here is the very long line of mechanical suture.
Regardless of the type of stapler, the length of staples, the use of additional methods to strengthen the suture line (e.g. running stitch, tissue adhesives or biological or synthetic buttressing materials), leakage into the peritoneal cavity is observed in some patients (usually less than 1%). This usually leads to diffuse peritonitis and septic shock, which is an immediate patient’s life-threatening condition.
Causes
It is usually difficult to identify the cause of a suture line leakage after a resection. If there has been a technical error or malfunction of the stapler, it usually manifests itself immediately and can be detected during the leakage test performed intraoperatively. In the case of a leakage that develops later after surgery (after several days or so), various mechanisms leading to impaired healing have been postulated. Undoubtedly, general factors such as vitamin and protein deficiencies (frequently occurring in obese patients), or chronic use of drugs that impair healing, such as steroids, immunosuppressants, or chemotherapeutics, play a role. An important role of local ischemia has also been suggested, which may result from the specific anatomical structure of the gastro-esophageal junction. It seems, however, that one of the most important and sometimes underestimated factors is the shape of the remnant of the stomach. If, due to a technical error, the tube created from the stomach was twisted or there is some kinking, a pressure zone is present causing resistance to the flow of contents through the stomach. This causes an increase in pressure above this area, which, transferring to the gastric wall and stretching the staple line, can tear it and cause leakage.
Diagnosis
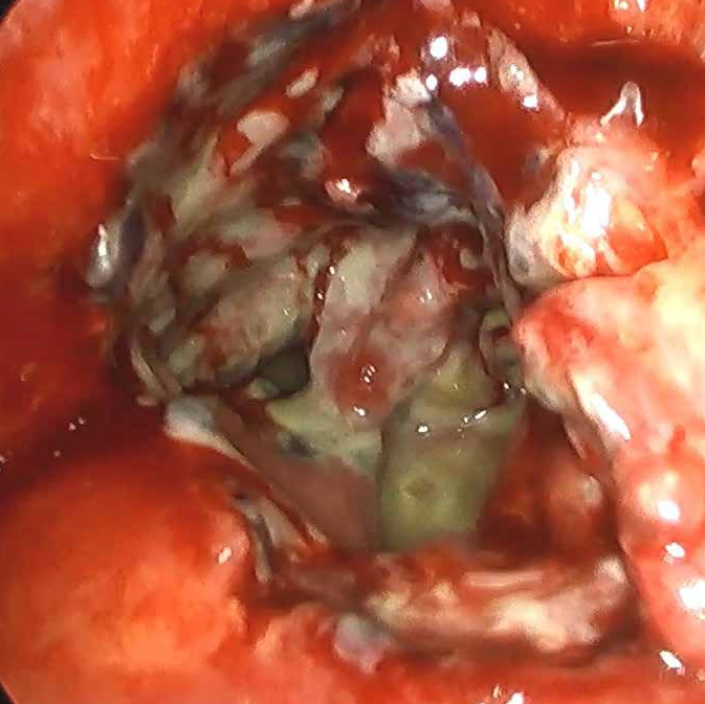
Photo 1: Laparoscopic image of a limited reservoir of gastric contents, near the leakage site. The leakage site itself was not visualized. Reoperation of the patient after laparoscopic sleeve gastrectomy
In order to achieve a favorable outcome of leakage treatment, it is crucial to diagnose the problem as early as possible and to begin treatment as soon as possible. Any delay leads to worsening of septic shock, up to and including irreversibility. Clinical assessment is most important. Symptoms of developing sepsis, abdominal pain, fever, tachycardia, deterioration of the patient’s general condition should always arouse concern and prompt further action. The diagnostic workout should not be postponed. None of the available methods allows for unequivocal the exclusion of leakage. Unless this significantly dose not significantly delays the decision about reoperation, some imaging studies such as upper gastrointestinal x-ray with contrasting agent or abdominal CT may be performed also after oral administration of contrasting agent. However, it should be remembered that these methods have a high rate of false-negative results. Inflammatory markers such as leukocytosis, C-reactive protein or procalcitonin may also be helpful. Regardless of the results of these tests, the only way to unequivocally resolve clinical doubts is reoperation and the decision about surgery should not be delayed. As the vast majority of bariatric procedures are now performed laparoscopically, the same technique should be applied for the reoperation. If no abdominal changes characteristic of suture line leakage are found, the surgery is limited to exploratory (diagnostic) laparoscopy, which is safe and well tolerated by the patient. The presence of intestinal contents, pus, inflammatory infiltration, fibrin deposits in the peritoneal cavity proves the leakage through the suture line and requires appropriate treatment (Photo 1).

Photo 1: Laparoscopic image of a limited reservoir of gastric contents, near the leakage site. The leakage site itself was not visualized. Reoperation of the patient after laparoscopic sleeve gastrectomy
Surgical treatment
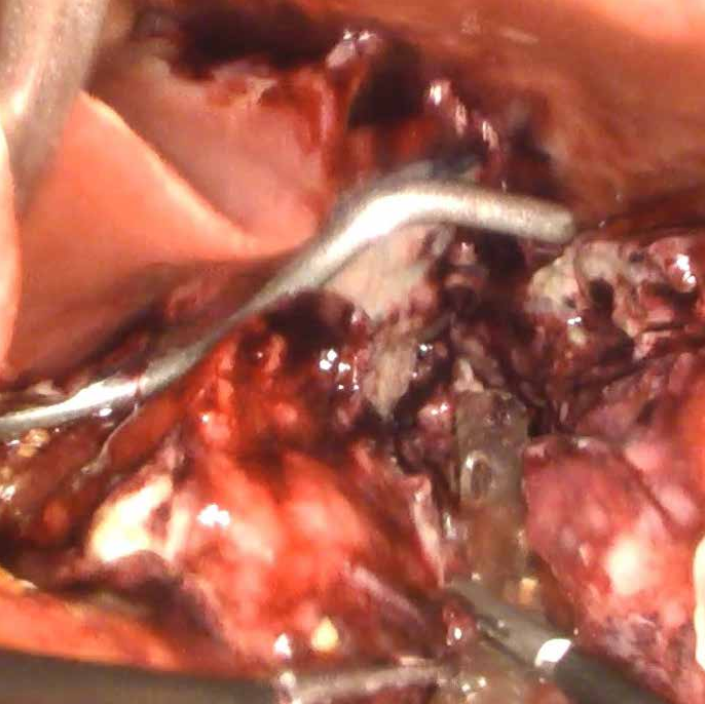
After sleeve gastrectomy, leakage is usually observed in the region of the GE junction. This site is difficult to access even in non-obese patients. However, in patients with morbid obesity, peritonitis causes important tissues edema and distention of intestinal loops which severely impairs the visualization of the leakage site. Blue dye infused through a nasogastric tube or intraoperative gastroscopy may be helpful in identification. Visualization of the leakage site is not critical to treatment. Even if the staple line dehiscence is relatively small and clearly visible, attempts to suture it are almost always unsuccessful. The inflamed tissues are not effectively approximated by sutures – they are usually very fragile and attempts at suturing usually lead only to a much larger defect. The laparoscopic procedure is therefore mainly aimed at a thorough lavage of the peritoneal cavity and insertion of a drain in the area of the leakage so that the gastric contents do not spill over the peritoneal cavity (Photo 2).
The successful surgical treatment leads to creating a fistula effectively draining leaking gastric content. In this situation all of the fluid from the gastrointestinal tract is effectively drained to the outside and such leakage is not accompanied by general symptoms of sepsis and the associated life-threatening condition. Over time, a tight channel of adhesion forms around the drain.
The key to successful treatment of a fistula, which is in fact created during laparoscopic surgery, is to create a lowpressure outflow for the contents of the gastrointestinal tract. The contents of the stomach and esophagus will pass according to the pressure gradient along the path of lesser resistance. If there is a stenosis, kinking or twisting in the distal part of the stomach below the fistula, the entire contents will flow through the fistula to the outside. The use of a fully coated self-expandable stent dedicated to the treatment of leakage after sleeve gastrectomy is helpful in creating such a low pressure outflow from the stomach.
The photographic material for this article was created during the treatment of a patient operated on in the Department of General and Oncological Surgery at Ludwik Rydygier Memorial Hospital in Cracow in February 2021. This patient was reoperated for suspected suture line leakage on day 4 after sleeve gastrectomy. During relaparoscopy the Hanaro ECBB/ ECBS stent (SEMS) was inserted.
Treatment technique
Early placement of this stent is extremely important. Since the beginning of my own experience with bariatric surgery, I try to place the stent intraoperatively, immediately after confirmation of the leakage of the suture line during relaparoscopy. Such a procedure allows for a quick and effective control of the septic shock, caused by further leakage of gastrointestinal contents. This requires the 24 hour availability of stents, endoscopy and a specialist able to perform this examination and stent placement during surgery, which, as is often the case in emergency surgery, is performed after “working hours”.
Stents for sleeve gastrectomy are usually quite long (over 20 cm) and larger in diameter than other stents. The proximal end of such a stent is widened in a funnel shape. They are fully coated with no anchoring features and, additionally, a lasso is woven into both ends which, when grasped with an endoscopic instrument and pulled up, causes the funnel of the stent to collapse and enables its removal after treatment. Such a stent should reach from the lower part of the esophagus, cover the leakage site and distal end should be positioned either just above the pylorus or in the duodenal bulb.
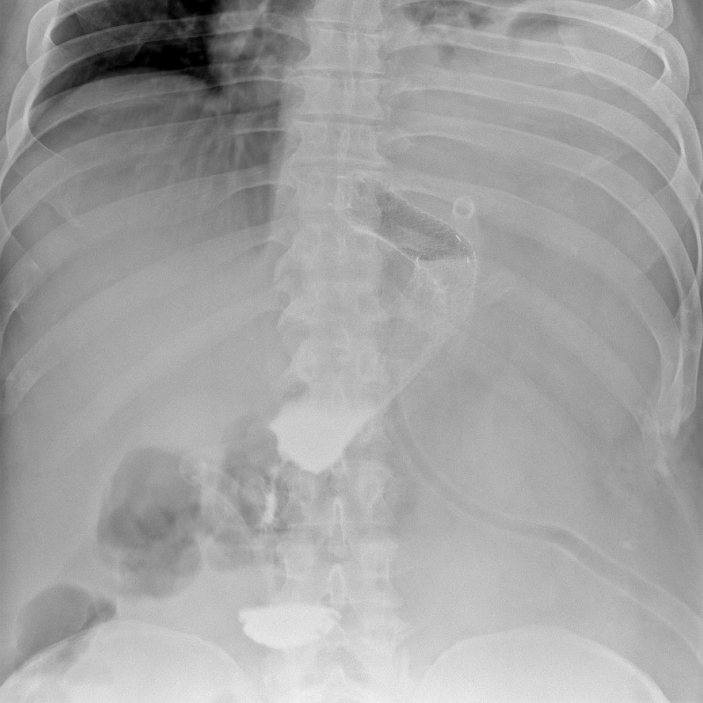
Its placement is technically quite difficult and requires a lot of experience. In the first stage, only an endoscope is inserted into the esophagus and stomach visualizing (together with laparoscopy) the site of the leakage, its location and size. It is usually possible to visualize also the obstruction to the flow of gastrointestinal contents below the leak site. This most often manifests itself in the narrowed lumen, which usually allows the endoscope to pass through, although with some difficulty. After the endoscope is passed below, into the duodenal bulb, a guidewire is placed through its working channel. The endoscope is then removed, and applicator with a stent is introduced over the guidewire. The position of the stent is determined by fluoroscopy (the procedure should be performed on an X-ray-transparent operating table) and a gastroscope placed parallel to the stent. Laparoscopic evaluation is also helpful if stent placement is performed during reoperation, which is the optimal procedure. The same methods are used to assess stent deployment. After gradual deployment of the stent, the proximal edge of the stent should be in the esophagus several centimeters above the gastric cardia (Photo 3). The distal one, on the other hand, should extend into the duodenum, or at least into the antrum of the stomach.
Gastroscopy should confirm that the distal end of the stent is not obstructed by the gastric wall, as this can lead to stent obstruction and failure. The stent can be re-positioned with the endoscope by pulling upwards or downwards with lassos woven into the proximal or distal end of the stent.
Follow-up treatment
Key to further treatment is the control of septic shock. All available methods implemented in modern intensive care units, where the patient should be initially treated, are applicable here, however a detailed discussion about them goes beyond the scope of this paper. Local treatment, on the other hand, requires a strong commitment from the endoscopic and surgical team. Output of drain contents left in the peritoneal cavity during relaparoscopy should be monitored. Ideally, the leakage should have resolved or should only happen through one of the drains closest to the leakage site. Effective drainage is essential for proper treatment. If the drain fails to drain, which is accompanied by increasing septic symptoms and inflammatory parameters, immediate intervention is required. It is usually a good idea to do a CT scan to confirm the location of the drain and the presence of any fluid collections or free intraperitoneal fluid. In some cases, it may be sufficient to simply flush and drain the drains. It is also possible to confirm effective drainage on X-ray examination by injecting a non-irritating contrasting agent into the drains. However, if good control of gastrointestinal outflow through the fistula cannot be restored and the patient develops septic symptoms, he/she should be submitted to reoperation, aimed at dissection of all the adhesions, drains should be unblocked or replaced and their position corrected. Laparoscopic reoperation, which is successful in the vast majority of patients, should also be attempted. In the case of the patient whose treatment inspired this article, this was necessary on day 12 after reoperation with stent implantation.
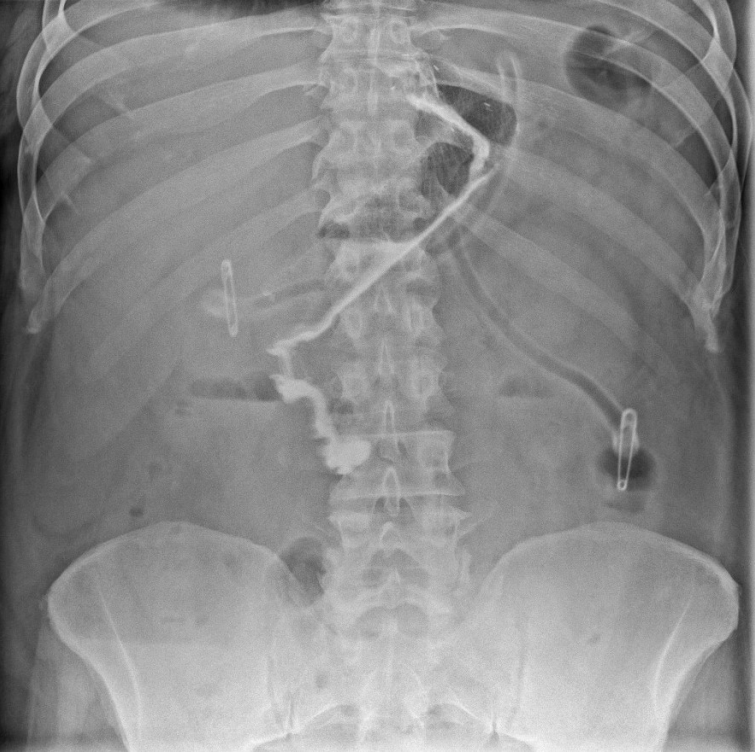
Fully coated stents for the treatment of leaks after bariatric surgery do not have anchoring systems. This is necessary as we plan to remove them at the end of treatment, usually not earlier than 6-8 weeks after implantation of such a stent. The flaw of such a system is undoubtedly the high intrinsic mobility of the stent, which can move both upwards and downwards. Therefore, frequent assessment of the stent’s location is necessary. Standard X-ray is usually performed, but endoscopy can also be used (Photo 4). In case of significant stent migration, the stent position should be corrected using the methods described above.
End of treatment
The treatment is considered successful if the patient’s general condition improves steadily, there is no accumulation of gastric content in the peritoneal cavity, the leakage through the drain left closest to the leakage site gradually decreases and finally stops (which is not accompanied by symptoms of sepsis and an increase in inflammatory markers). In such a situation, the oral supply initially of clear fluids followed by liquid diet may be introduced. Gradually, all drains are removed from the peritoneal cavity, leaving the fistula drain for the longest time. If there is no significant content secretion through the fistula, we begin a slow, multi-stage removal, systematically pulling it up by several centimeters every few days.
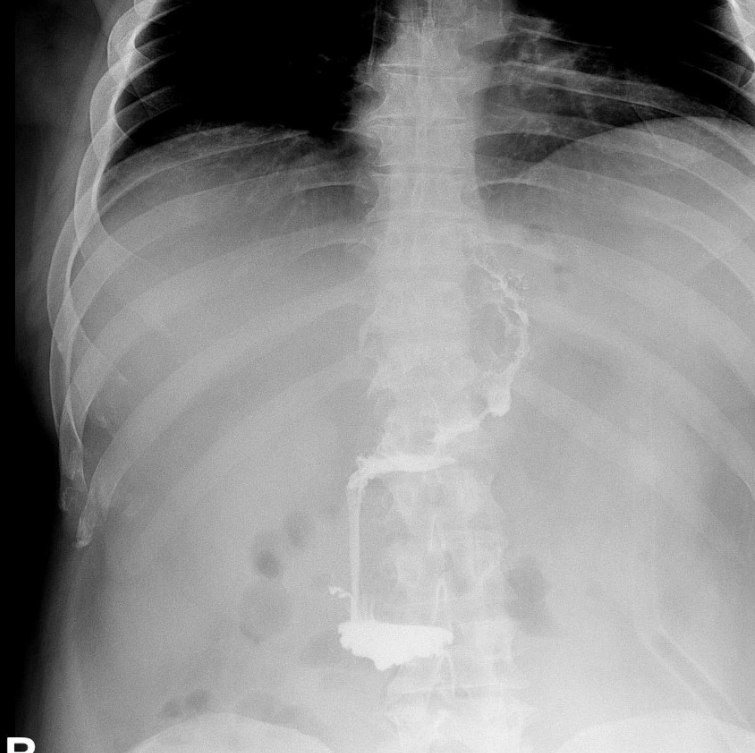
We usually confirm normal flow through the upper gastrointestinal tract by fluoroscopy before stent removal (Photo 5). It is equally important to confirm that there is no leakage of contrasting agent outside the gastrointestinal lumen. The same examination is performed again after endoscopic removal of the stent (Photo 6). If the patient tolerates the oral diet well, in the absence of general symptoms, the treatment can be considered completed after a few days of observation.
Author
Prof. Andrzej Budzyński, MD, PhD, Head of General and Oncological Surgery Unit, Specialized Hospital – Ludwig Rydygier Memorial Hospital in Cracow, Poland
- Keyword
- Content Type