HANAROSTENT Fully Covered Esophageal Stent
Choosing the Correct Stent for the Management of Complex Esophageal Strictures
Authors

Endoscopy Unit of the Cancer Institute of São Paulo—ICESP Department of Gastroenterology of the University of São Paulo
Associate Editor for Gastrointestinal Endoscopy

Endoscopy Unit of the Cancer Institute of São Paulo—ICESP Department of Gastroenterology of the University of São Paulo
Case presentation
A 52-year-old male with a history of alcohol and tobacco use was admitted to a healthcare facility in June 2016 with abrupt onset of dysphagia. Endoscopy revealed a food bolus impacted in a malignant stricture caused by squamous cell carcinoma. A partially covered (PC) 18 mm x 105 mm self-expandable metal stent (SEMS) was placed during the same procedure.
Case management
Five months after the original PC SEMS placement, the patient presented with a recurrence of dysphagia. The patient was subsequently referred to the Endoscopy Unit of the Cancer Institute of São Paulo for oncologic
evaluation. At staging, lymph nodes were found at the celiac trunk and in close proximity to the aortic arch and trachea.
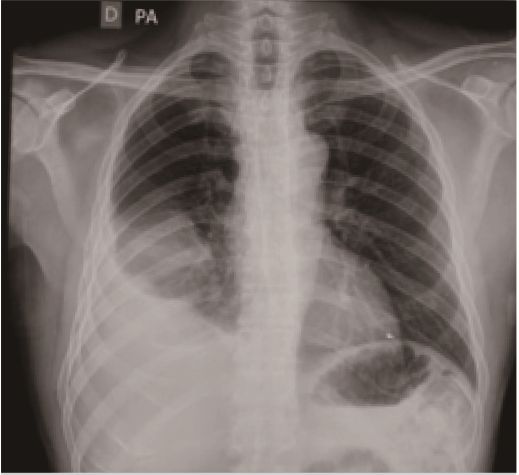
Although the referring facility did not provide endoscopic or radiologic images from the first intervention, a thoracic x-ray performed by São Paulo showed the previous SEMS
in place (Figures 1 and 2).
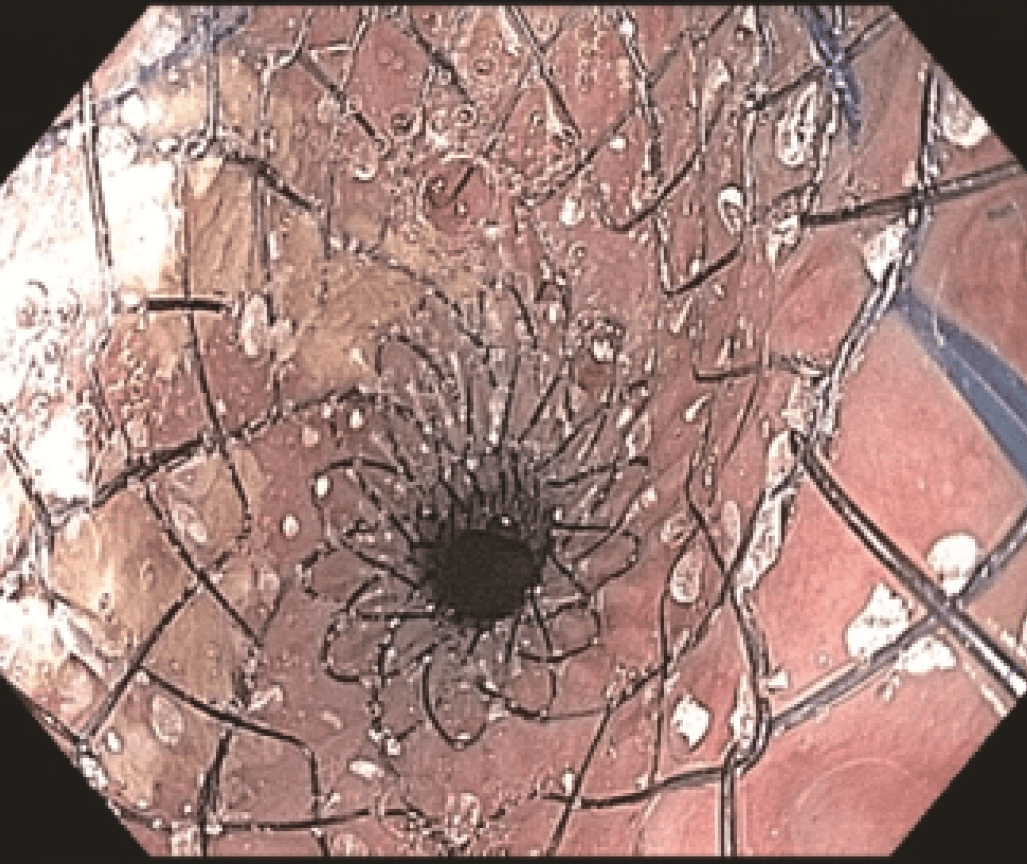
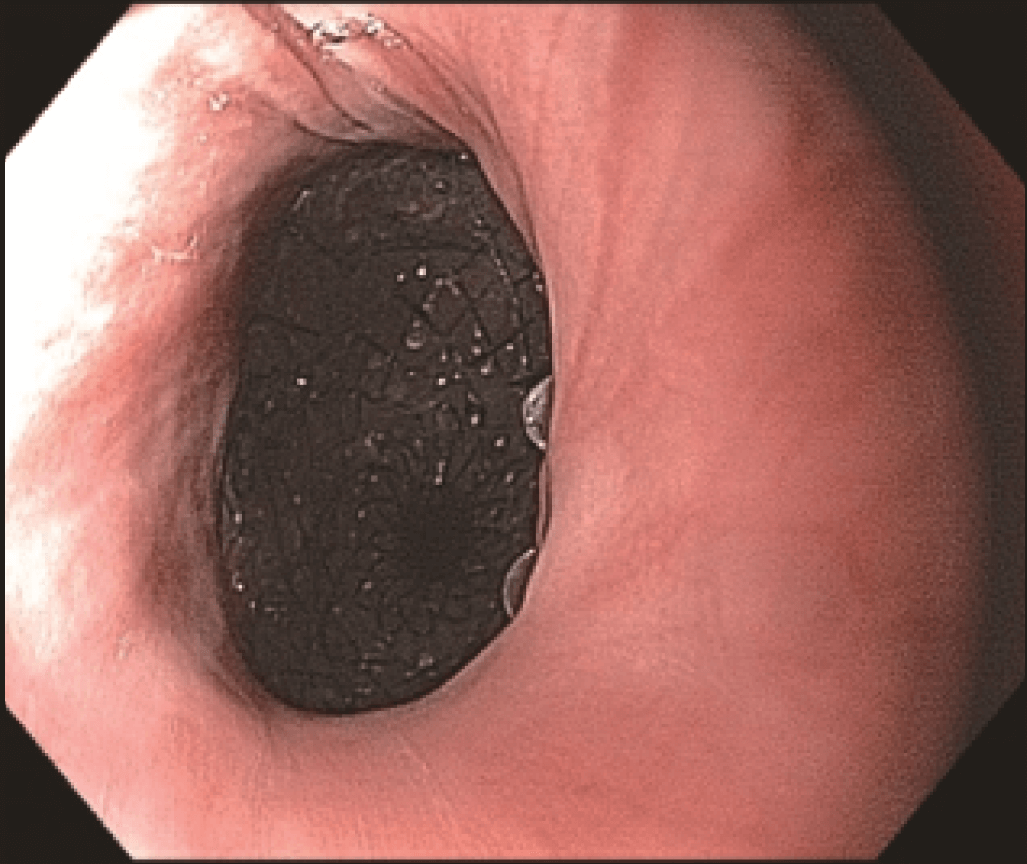
Endoscopic evaluation identified a stricture located at the proximal end of the SEMS (Figures 3 and 4) at 20 cm from the incisors, apparently caused by tumor growth. The upper esophageal sphincter was located at 16 cm from the incisors.
The stricture was located at the uncovered proximal end of the initial SEMS, placed at the cervical esophagus. The stricture was hard and did not allow the passage of the 4.9 mm scope.
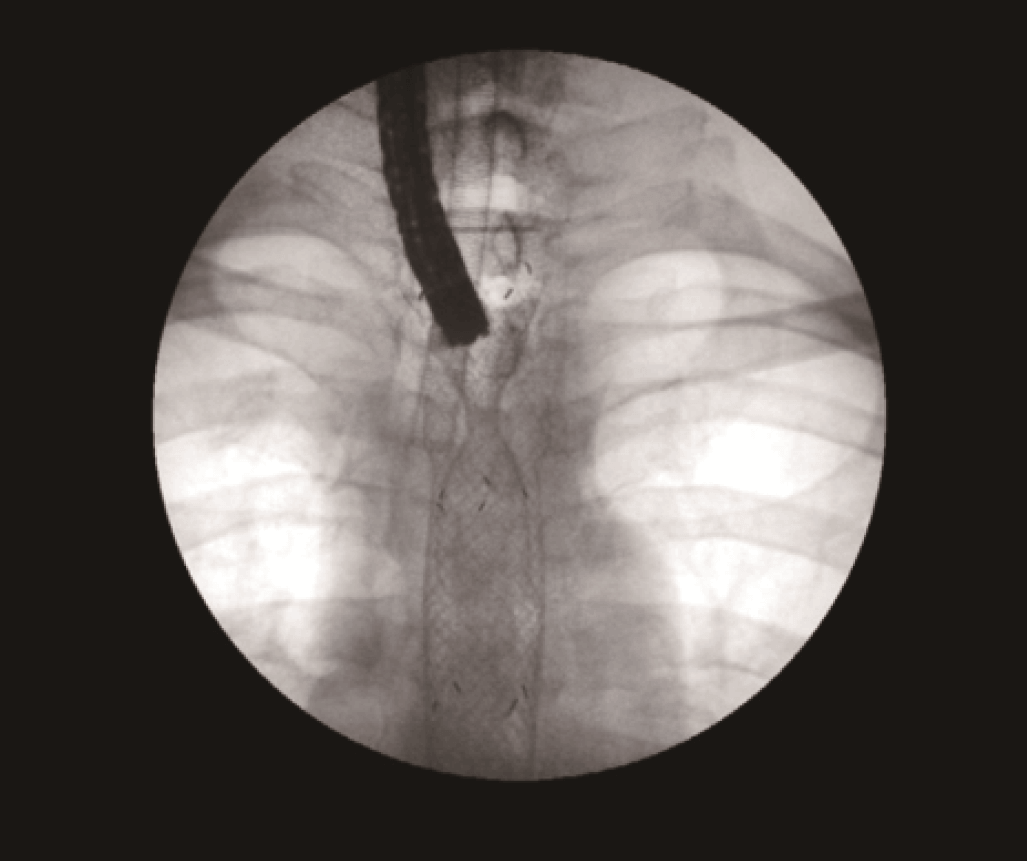
To open the stricture, an 18 mm x 80 mm fully covered esophageal HANAROSTENT was introduced. The accurate placement of SEMS in the proximal esophagus is usually challenging.
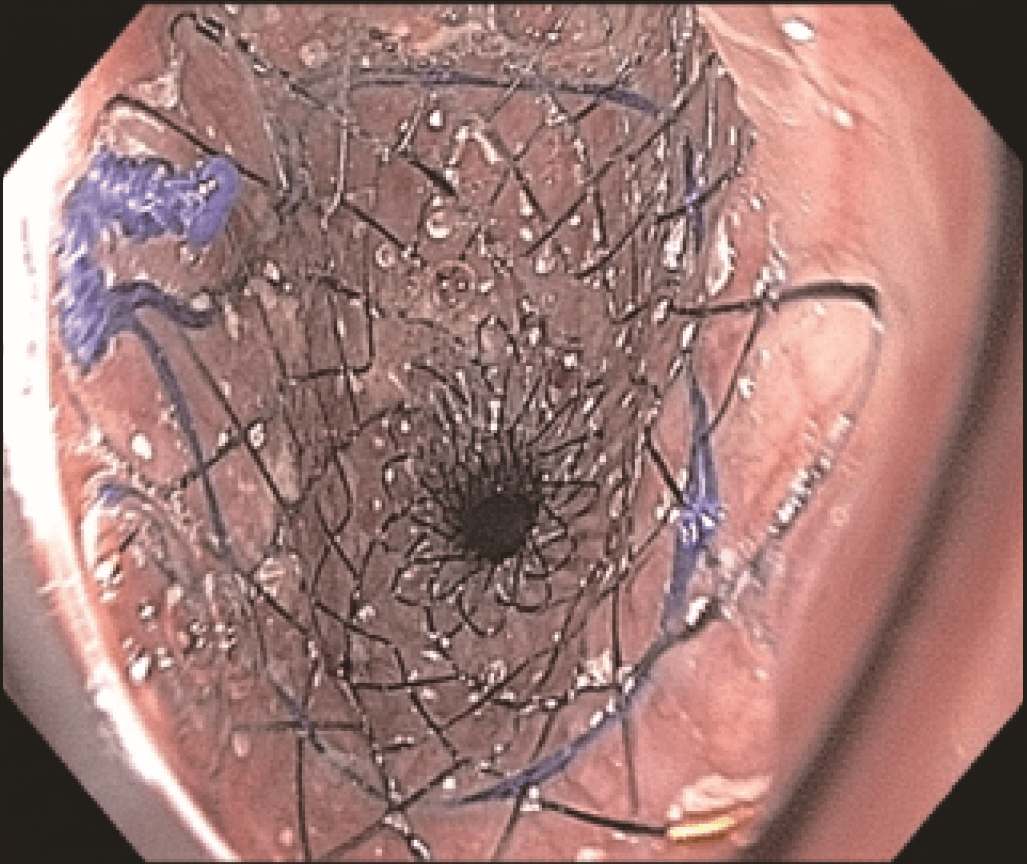
However, the three marks at the HANAROSTENT’s distal, medial and proximal ends were easily seen under fluoroscopic guidance, and were valuable in ensuring acurate deployment, just distal to the upper esophageal sphincter (Figure 5). The distal end of the HANAROSTENT remained in the patent portion of the previous stent. The endoscopic view of the SEMS proximal end can be observed in the images below (Figures 6, 7 and 8).
Conclusion
Both deployment and patency of the fully covered esophageal HANAROSTENT were very good. The flexibility and softness of the SEMS contributed to the patient’s ability to tolerate a stent placed in the proximal esophagus.
About HANAROSTENT
The 510(k)-cleared HANAROSTENT® Esophagus is made by M.I.Tech and distributed exclusively through Olympus in the U.S. and EU market. The stent is designed to allow for appropriate radial and axial force, reduce the risk of migration, and provide accurate placement. The device is available in 8 sizes to meet an array of therapeutic requirements.
- Content Type